Chopping off the chondrocyte proteome
- PMID: 25179281
- PMCID: PMC4819584
- DOI: 10.3109/1354750X.2014.955884
Chopping off the chondrocyte proteome
Abstract
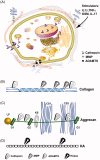
The progressive nature of osteoarthritis is manifested by the dynamic increase of degenerated articular cartilage, which is one of the major characteristics of this debilitating disease. As articular chondrocytes become exposed to inflammatory stress they enter a pro-catabolic state, which leads to the secretion and activation of a plethora of proteases. In aim to detect the disease before massive areas of cartilage are destroyed, various protein and non-protein biomarkers have been examined in bodily fluids and correlated with disease severity. This review will discuss the widely research extracellular degraded products as well as products generated by affected cellular pathways upon increased protease activity. While extracellular components could be more abundant, cleaved cellular proteins are less abundant and are suggested to possess a significant effect on cell metabolism and cartilage secretome. Subtle changes in cell secretome could potentially act as indicators of the chondrocyte metabolic and biological state. Therefore, it is envisioned that combined biomarkers composed of both cell and extracellular-degraded secretome could provide a valuable platform for testing drug efficacy to halt disease progression at its early stages.
Keywords: Biomarkers; chondrocytes; osteoarthritis; proteolytic enzymes; proteome.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
