Defective channels lead to an impaired skin barrier
- PMID: 25179597
- PMCID: PMC4197083
- DOI: 10.1242/jcs.154633
Defective channels lead to an impaired skin barrier
Abstract
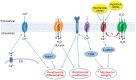
Channels are integral membrane proteins that form a pore, allowing the passive movement of ions or molecules across a membrane (along a gradient), either between compartments within a cell, between intracellular and extracellular environments or between adjacent cells. The ability of cells to communicate with one another and with their environment is a crucial part of the normal physiology of a tissue that allows it to carry out its function. Cell communication is particularly important during keratinocyte differentiation and formation of the skin barrier. Keratinocytes in the skin epidermis undergo a programme of apoptosis-driven terminal differentiation, whereby proliferating keratinocytes in the basal (deepest) layer of the epidermis stop proliferating, exit the basal layer and move up through the spinous and granular layers of the epidermis to form the stratum corneum, the external barrier. Genes encoding different families of channel proteins have been found to harbour mutations linked to a variety of rare inherited monogenic skin diseases. In this Commentary, we discuss how human genetic findings in aquaporin (AQP) and transient receptor potential (TRP) channels reveal different mechanisms by which these channel proteins function to ensure the proper formation and maintenance of the skin barrier.
Keywords: Aquaporin; Gap junction; TRP channel.
© 2014. Published by The Company of Biologists Ltd.
Figures

References
-
- Bakirtzis G., Choudhry R., Aasen T., Shore L., Brown K., Bryson S., Forrow S., Tetley L., Finbow M., Greenhalgh D. et al. (2003). Targeted epidermal expression of mutant Connexin 26(D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum. Mol. Genet. 12, 1737–1744 10.1093/hmg/ddg183 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

