Easy method to examine single nerve fiber excitability and conduction parameters using intact nonanesthetized earthworms
- PMID: 25179616
- PMCID: PMC4154267
- DOI: 10.1152/advan.00137.2013
Easy method to examine single nerve fiber excitability and conduction parameters using intact nonanesthetized earthworms
Abstract
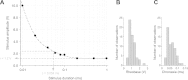
The generation and conduction of neuronal action potentials (APs) were the subjects of a cell physiology exercise for first-year medical students. In this activity, students demonstrated the all-or-none nature of AP generation, measured conduction velocity, and examined the dependence of the threshold stimulus amplitude on stimulus duration. For this purpose, they used the median giant nerve fiber (MGF) in the ventral nerve cord of the common earthworm (Lumbricus terrestris). Here, we introduce a specialized stimulation and recording chamber that the nonanesthetized earthworm enters completely unforced. The worm resides in a narrow round duct with silver electrodes on the bottom such that individual APs of the MGF can be elicited and recorded superficially. Our experimental setup combines several advantages: it allows noninvasive single fiber AP measurements taken from a nonanesthetized animal that is yet restrained. Students performed the experiments with a high success rate. According to the data acquired by the students, the mean conduction velocity of the MGF was 30.2 m/s. From the amplitude-duration relationship for threshold stimulation, rheobase and chronaxie were graphically determined by the students according to Lapicque's method. The mean rheobase was 1.01 V, and the mean chronaxie was 0.06 ms. The acquired data and analysis results are of high quality, as deduced from critical examination based on the law of Weiss. In addition, we provide video material, which was also used in the practical course.
Keywords: Lapicque; Weiss; action potential; chronaxie; conduction velocity; extracellular recording; rheobase.
Copyright © 2014 The American Physiological Society.
Figures




Similar articles
-
Electrophysiological correlates of rapid escape reflexes in intact earthworms, Eisenia foetida. I. Functional development of giant nerve fibers during embryonic and postembryonic periods.J Neurobiol. 1982 Jul;13(4):337-53. doi: 10.1002/neu.480130405. J Neurobiol. 1982. PMID: 7108516
-
Determination of electrode to nerve fiber distance and nerve conduction velocity through spectral analysis of the extracellular action potentials recorded from earthworm giant fibers.Med Biol Eng Comput. 2012 Aug;50(8):867-75. doi: 10.1007/s11517-012-0930-8. Epub 2012 Jun 20. Med Biol Eng Comput. 2012. PMID: 22714669
-
Impulse conduction in the myelinated giant fibers of the earthworm. Structure and function of the dorsal nodes in the median giant fiber.J Comp Neurol. 1976 Aug 15;168(4):505-31. doi: 10.1002/cne.901680405. J Comp Neurol. 1976. PMID: 939820
-
[Test for analysing nerve conduction velocity].Rinsho Shinkeigaku. 1991 Dec;31(12):1326-9. Rinsho Shinkeigaku. 1991. PMID: 1817800 Review. Japanese.
-
Efferent and afferent fibres in human sacral ventral nerve roots: basic research and clinical implications.Electromyogr Clin Neurophysiol. 1989 Jan-Feb;29(1):33-53. Electromyogr Clin Neurophysiol. 1989. PMID: 2649371 Review.
Cited by
-
Integrating Programming into Neuroscience Courses.J Undergrad Neurosci Educ. 2024 Jul 21;22(2):A99-A103. doi: 10.59390/PYYP5010. eCollection 2024 Winter. J Undergrad Neurosci Educ. 2024. PMID: 39280707 Free PMC article.
References
-
- Bear MF, Connors BW, Paradiso MA. Neuroscience. Baltimore, MD: Lippincott, Williams & Wilkins, 2007.
-
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 21: 137–158, 1998. - PubMed
-
- Brismar T. Electrical properties of isolated demyelinated rat nerve fibres. Acta Physiol Scand 113: 161–166, 1981. - PubMed
-
- Bullock TH. Functional organization of the giant fiber system of lumbricus. J Neurophysiol 8: 55–71, 1945.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

