Assemblages: functional units formed by cellular phase separation
- PMID: 25179628
- PMCID: PMC4151146
- DOI: 10.1083/jcb.201404124
Assemblages: functional units formed by cellular phase separation
Abstract
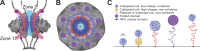
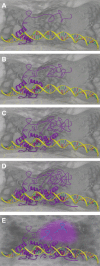
The partitioning of intracellular space beyond membrane-bound organelles can be achieved with collections of proteins that are multivalent or contain low-complexity, intrinsically disordered regions. These proteins can undergo a physical phase change to form functional granules or other entities within the cytoplasm or nucleoplasm that collectively we term "assemblage." Intrinsically disordered proteins (IDPs) play an important role in forming a subset of cellular assemblages by promoting phase separation. Recent work points to an involvement of assemblages in disease states, indicating that intrinsic disorder and phase transitions should be considered in the development of therapeutics.
© 2014 Toretsky and Wright.
Figures

References
-
- Aggarwal, S., Snaidero N., Pähler G., Frey S., Sánchez P., Zweckstetter M., Janshoff A., Schneider A., Weil M.T., Schaap I.A., et al. . 2013. Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biol. 11:e1001577 10.1371/journal.pbio.1001577 - DOI - PMC - PubMed
-
- Ano Bom, A.P., Rangel L.P., Costa D.C., de Oliveira G.A., Sanches D., Braga C.A., Gava L.M., Ramos C.H., Cepeda A.O., Stumbo A.C., et al. . 2012. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils: implications for cancer. J. Biol. Chem. 287:28152–28162 10.1074/jbc.M112.340638 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

