Selective neuronal lineages derived from Dll4-expressing progenitors/precursors in the retina and spinal cord
- PMID: 25179941
- PMCID: PMC4276465
- DOI: 10.1002/dvdy.24185
Selective neuronal lineages derived from Dll4-expressing progenitors/precursors in the retina and spinal cord
Abstract
Background: During retinal and spinal cord neurogenesis, Notch signaling plays crucial roles in regulating proliferation and differentiation of progenitor cells. One of the Notch ligands, Delta-like 4 (Dll4), has been shown to be expressed in subsets of retinal and spinal cord progenitors/precursors and involved in neuronal subtype specification. However, it remains to be determined whether Dll4 expression has any progenitor/precursor-specificity contributing to its functional specificity during neural development.
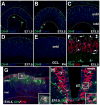
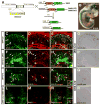
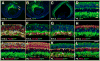
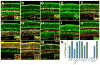
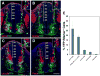
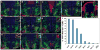
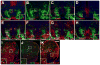
Results: We generated a Dll4-Cre BAC transgenic mouse line that drives Cre recombinase expression mimicking that of the endogenous Dll4 in the developing retina and spinal cord. By fate-mapping analysis, we found that Dll4-expressing progenitors/precursors give rise to essentially all cone, amacrine and horizontal cells, a large portion of rod and ganglion cells, but only few bipolar and Müller cells. In the spinal cord, Dll4-expressing progenitors/precursors generate almost all V2a and V2c cells while producing only a fraction of the cells for other interneuron and motor neuron subtypes along the dorsoventral axis.
Conclusions: Our data suggest that selective expression of Dll4 in progenitors/precursors contributes to its functional specificity in neuronal specification and that the Dll4-Cre line is a valuable tool for gene manipulation to study Notch signaling.
Keywords: Dll4; Notch signaling; V2 interneuron; neuronal lineage; retina; spinal cord.
© 2014 Wiley Periodicals, Inc.
Figures

References
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
-
- Baas D, Bumsted KM, Martinez JA, Vaccarino FM, Wikler KC, Barnstable CJ. The subcellular localization of Otx2 is cell-type specific and developmentally regulated in the mouse retina. Brain Res Mol Brain Res. 2000;78:26–37. - PubMed
-
- Benedito R, Duarte A. Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr Patterns. 2005;5:750–755. - PubMed
-
- Billiard F, Kirshner JR, Tait M, Danave A, Taheri S, Zhang W, Waite JC, Olson K, Chen G, Coetzee S, Hylton D, Murphy AJ, Yancopoulos GD, Thurston G, Skokos D. Ongoing Dll4-Notch signaling is required for T-cell homeostasis in the adult thymus. Eur J Immunol. 2011;41:2207–2216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

