P2Y(12) receptor on the verge of a neuroinflammatory breakdown
- PMID: 25180027
- PMCID: PMC4142314
- DOI: 10.1155/2014/975849
P2Y(12) receptor on the verge of a neuroinflammatory breakdown
Abstract
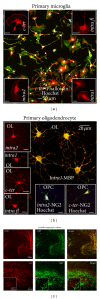
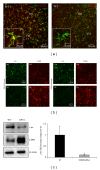
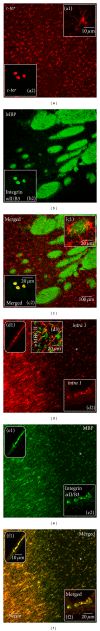
In the CNS, neuroinflammation occurring during pathologies as amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) is the consequence of an intricate interplay orchestrated by various cell phenotypes. Among the molecular cues having a role in this process, extracellular nucleotides are responsible for intercellular communication and propagation of inflammatory stimuli. This occurs by binding to several receptor subtypes, defined P2X/P2Y, which are widespread in different tissues and simultaneously localized on multiple cells. For instance, the metabotropic P2Y12 subtype is found in the CNS on microglia, affecting activation and chemotaxis, on oligodendrocytes, possessing a hypothesized role in myelination, and on astrocytes. By comparative analysis, we have established here that P2Y12 receptor immunolabelled by antibodies against C-terminus or second intracellular loop, is, respectively, distributed and modulated under neuroinflammatory conditions on ramified microglia or myelinated fibers, in primary organotypic cerebellar cultures, tissue slices from rat striatum and cerebellum, spinal cord sections from symptomatic/end stage SOD1-G93A ALS mice, and finally autoptic cortical tissue from progressive MS donors. We suggest that modulation of P2Y12 expression might play a dual role as analytic marker of branched/surveillant microglia and demyelinating lesions, thus potentially acquiring a predictive value under neuroinflammatory conditions as those found in ALS and MS.
Figures




References
-
- Guérout N, Li X, Barnabé-Heider F. Cell fate control in the developing central nervous system. Experimental Cell Research. 2014;321(1):77–83. - PubMed
-
- Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. The Lancet Neurology. 2011;10(3):253–263. - PubMed
-
- Ellwardt E, Zipp F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Experimental Neurology. 2014 - PubMed
-
- Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nature Reviews Neuroscience. 2014;15(2):84–97. - PubMed
-
- di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends in Neurosciences. 2009;32(2):79–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

