Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux
- PMID: 25180079
- PMCID: PMC4837999
- DOI: 10.1093/ntr/ntu175
Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux
Abstract
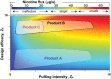
Electronic cigarettes (ECIGs) comprise an aerosolized nicotine delivery product category that provides consumers with probably unprecedented control over extensive features and operating conditions, allowing a wide range of nicotine yields to be obtained. Depending on the combination of such ECIG variables as electrical power input, geometry, liquid composition, and puff behavior, ECIG users can extract in a few puffs far more or far less nicotine than with a conventional combustible cigarette. These features of ECIG design and use present challenges for public health policy, central among which is the question of how to regulate nicotine delivery. In this commentary, we propose a conceptual framework intended to provide a convenient approach for evaluating and regulating the nicotine emitted from ECIGs. This framework employs nicotine flux to account for the total dose and rate at which nicotine reaches the user, 2 key factors in drug abuse liability. The nicotine flux is the nicotine emitted per puff second (e.g., mg/s) by a given ECIG design under given use conditions, and it can be predicted accurately using physical principles. We speculate that if the flux is too low, users likely will abandon the device and maintain conventional tobacco product use. Also, we speculate that if the flux is too high, individuals may suffer toxic side effects and/or the device may have higher-than-necessary abuse liability. By considering ECIG design, operation conditions, liquid composition, and puff behavior variables in combination, we illustrate how ECIG specifications can be realistically mandated to result in a target flux range.
© The Author 2014. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Nicotine flux: a potentially important tool for regulating electronic cigarettes.Nicotine Tob Res. 2015 Feb;17(2):165-7. doi: 10.1093/ntr/ntu208. Epub 2014 Oct 19. Nicotine Tob Res. 2015. PMID: 25332456 Free PMC article. No abstract available.
-
Risks of attempting to regulate nicotine flux in electronic cigarettes.Nicotine Tob Res. 2015 Feb;17(2):163-4. doi: 10.1093/ntr/ntu207. Epub 2014 Oct 19. Nicotine Tob Res. 2015. PMID: 25332460 Free PMC article. No abstract available.
References
-
- Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2012;15:158–166. - PubMed
-
- Westenberger BJ. Evaluation of E-cigarettes. Rockville, MD: Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Division of Pharmaceutical Analysis; 2009. www.fda.gov/downloads/drugs/scienceresearch/ucm173250.pdf Accessed May 15, 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

