Immunologic effects of hydroxyurea in sickle cell anemia
- PMID: 25180279
- PMCID: PMC4179098
- DOI: 10.1542/peds.2014-0571
Immunologic effects of hydroxyurea in sickle cell anemia
Abstract
Background and objective: Susceptibility to encapsulated bacteria is well known in sickle cell disease (SCD). Hydroxyurea use is common in adults and children with SCD, but little is known about hydroxyurea's effects on immune function in SCD. Because hydroxyurea inhibits ribonucleotide reductase, causing cell cycle arrest at the G1-S interface, we postulated that hydroxyurea might delay transition from naive to memory T cells, with inhibition of immunologic maturation and vaccine responses.
Methods: T-cell subsets, naive and memory T cells, and antibody responses to pneumococcal and measles, mumps, and rubella vaccines were measured among participants in a multicenter, randomized, double-blind, placebo-controlled trial of hydroxyurea in infants and young children with SCD (BABY HUG).
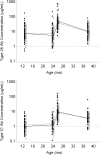
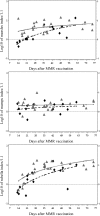
Results: Compared with placebo, hydroxyurea treatment resulted in significantly lower total lymphocyte, CD4, and memory T-cell counts; however, these numbers were still within the range of historical healthy controls. Antibody responses to pneumococcal vaccination were not affected, but a delay in achieving protective measles antibody levels occurred in the hydroxyurea group. Antibody levels to measles, mumps, and rubella showed no differences between groups at exit, indicating that effective immunization can be achieved despite hydroxyurea use.
Conclusions: Hydroxyurea does not appear to have significant deleterious effects on the immune function of infants and children with SCD. Additional assessments of lymphocyte parameters of hydroxyurea-treated children may be warranted. No changes in current immunization schedules are recommended; however, for endemic disease or epidemics, adherence to accelerated immunization schedules for the measles, mumps, and rubella vaccine should be reinforced.
Keywords: hydroxyurea; immunology; sickle cell disease; vaccines.
Copyright © 2014 by the American Academy of Pediatrics.
Figures


References
-
- Brawley OW, Cornelius LJ, Edwards LR, et al. . National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med. 2008;148(12):932–938 - PubMed
-
- Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore). 1971;50(2):97–112 - PubMed
-
- Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. . Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr. 2003;143(4):438–444 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

