Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis
- PMID: 25180604
- PMCID: PMC4191002
- DOI: 10.1172/JCI75212
Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis
Abstract
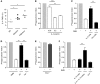
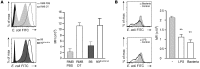
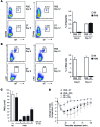
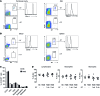
Controlling the overwhelming inflammatory reaction associated with polymicrobial sepsis remains a prevalent clinical challenge with few treatment options. In septic peritonitis, blood neutrophils and monocytes are rapidly recruited into the peritoneal cavity to control infection, but the role of resident sentinel cells during the early phase of infection is less clear. In particular, the influence of mast cells on other tissue-resident cells remains poorly understood. Here, we developed a mouse model that allows both visualization and conditional ablation of mast cells and basophils to investigate the role of mast cells in severe septic peritonitis. Specific depletion of mast cells led to increased survival rates in mice with acute sepsis. Furthermore, we determined that mast cells impair the phagocytic action of resident macrophages, thereby allowing local and systemic bacterial proliferation. Mast cells did not influence local recruitment of neutrophils and monocytes or the release of inflammatory cytokines. Phagocytosis inhibition by mast cells involved their ability to release prestored IL-4 within 15 minutes after bacterial encounter, and treatment with an IL-4-neutralizing antibody prevented this inhibitory effect and improved survival of septic mice. Our study uncovers a local crosstalk between mast cells and macrophages during the early phase of sepsis development that aggravates the outcome of severe bacterial infection.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

