Oral microbiome link to neurodegeneration in glaucoma
- PMID: 25180891
- PMCID: PMC4152129
- DOI: 10.1371/journal.pone.0104416
Oral microbiome link to neurodegeneration in glaucoma
Abstract
Background: Glaucoma is a progressive optic nerve degenerative disease that often leads to blindness. Local inflammatory responses are implicated in the pathology of glaucoma. Although inflammatory episodes outside the CNS, such as those due to acute systemic infections, have been linked to central neurodegeneration, they do not appear to be relevant to glaucoma. Based on clinical observations, we hypothesized that chronic subclinical peripheral inflammation contributes to neurodegeneration in glaucoma.
Methods: Mouthwash specimens from patients with glaucoma and control subjects were analyzed for the amount of bacteria. To determine a possible pathogenic mechanism, low-dose subcutaneous lipopolysaccharide (LPS) was administered in two separate animal models of glaucoma. Glaucomatous neurodegeneration was assessed in the retina and optic nerve two months later. Changes in gene expression of toll-like receptor 4 (TLR4) signaling pathway and complement as well as changes in microglial numbers and morphology were analyzed in the retina and optic nerve. The effect of pharmacologic blockade of TLR4 with naloxone was determined.
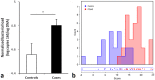
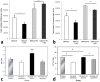
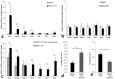
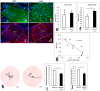
Findings: Patients with glaucoma had higher bacterial oral counts compared to control subjects (p<0.017). Low-dose LPS administration in glaucoma animal models resulted in enhancement of axonal degeneration and neuronal loss. Microglial activation in the optic nerve and retina as well as upregulation of TLR4 signaling and complement system were observed. Pharmacologic blockade of TLR4 partially ameliorated the enhanced damage.
Conclusions: The above findings suggest that the oral microbiome contributes to glaucoma pathophysiology. A plausible mechanism by which increased bacterial loads can lead to neurodegeneration is provided by experiments in animal models of the disease and involves activation of microglia in the retina and optic nerve, mediated through TLR4 signaling and complement upregulation. The finding that commensal bacteria may play a role in the development and/or progression of glaucomatous pathology may also be relevant to other chronic neurodegenerative disorders.
Conflict of interest statement
Figures
References
-
- Stasi K, Nagel D, Yang X, Wang RF, Ren L, et al. (2006) Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci 47: 1024–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

