Mechanisms for enzymatic cleavage of the N-glycosidic bond in DNA
- PMID: 25181003
- PMCID: PMC4238931
- DOI: 10.1039/c4ob01063a
Mechanisms for enzymatic cleavage of the N-glycosidic bond in DNA
Abstract
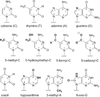
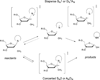
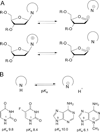
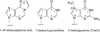
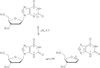
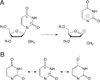
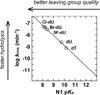
DNA glycosylases remove damaged or enzymatically modified nucleobases from DNA, thereby initiating the base excision repair (BER) pathway, which is found in all forms of life. These ubiquitous enzymes promote genomic integrity by initiating repair of mutagenic and/or cytotoxic lesions that arise continuously due to alkylation, deamination, or oxidation of the normal bases in DNA. Glycosylases also perform essential roles in epigenetic regulation of gene expression, by targeting enzymatically-modified forms of the canonical DNA bases. Monofunctional DNA glycosylases hydrolyze the N-glycosidic bond to liberate the target base, while bifunctional glycosylases mediate glycosyl transfer using an amine group of the enzyme, generating a Schiff base intermediate that facilitates their second activity, cleavage of the DNA backbone. Here we review recent advances in understanding the chemical mechanism of monofunctional DNA glycosylases, with an emphasis on how the reactions are influenced by the properties of the nucleobase leaving-group, the moiety that varies across the vast range of substrates targeted by these enzymes.
Figures

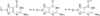

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

