Synthesis of non-linear protein dimers through a genetically encoded Thiol-ene reaction
- PMID: 25181502
- PMCID: PMC4152134
- DOI: 10.1371/journal.pone.0105467
Synthesis of non-linear protein dimers through a genetically encoded Thiol-ene reaction
Abstract
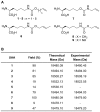
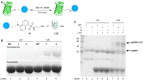
Site-specific incorporation of bioorthogonal unnatural amino acids into proteins provides a useful tool for the installation of specific functionalities that will allow for the labeling of proteins with virtually any probe. We demonstrate the genetic encoding of a set of alkene lysines using the orthogonal PylRS/PylTCUA pair in Escherichia coli. The installed double bond functionality was then applied in a photoinitiated thiol-ene reaction of the protein with a fluorescent thiol-bearing probe, as well as a cysteine residue of a second protein, showing the applicability of this approach in the formation of heterogeneous non-linear fused proteins.
Conflict of interest statement
Figures

Similar articles
-
The mycotoxin patulin induces intra- and intermolecular protein crosslinks in vitro involving cysteine, lysine, and histidine side chains, and alpha-amino groups.Chem Biol Interact. 1999 Nov 30;123(2):85-103. doi: 10.1016/s0009-2797(99)00123-4. Chem Biol Interact. 1999. PMID: 10597903
-
Introducing bioorthogonal functionalities into proteins in living cells.Acc Chem Res. 2011 Sep 20;44(9):742-51. doi: 10.1021/ar200067r. Epub 2011 Jun 2. Acc Chem Res. 2011. PMID: 21634380
-
Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction.Nat Chem. 2012 Feb 5;4(4):298-304. doi: 10.1038/nchem.1250. Nat Chem. 2012. PMID: 22437715 Free PMC article.
-
Thiol-yne radical reaction mediated site-specific protein labeling via genetic incorporation of an alkynyl-L-lysine analogue.Org Biomol Chem. 2013 Apr 28;11(16):2624-9. doi: 10.1039/c3ob27116a. Org Biomol Chem. 2013. PMID: 23450369
-
tRNAPyl: Structure, function, and applications.RNA Biol. 2018;15(4-5):441-452. doi: 10.1080/15476286.2017.1356561. Epub 2017 Sep 13. RNA Biol. 2018. PMID: 28837402 Free PMC article. Review.
Cited by
-
Incorporation of Amino Acids with Long-Chain Terminal Olefins into Proteins.Molecules. 2016 Feb 29;21(3):287. doi: 10.3390/molecules21030287. Molecules. 2016. PMID: 26938510 Free PMC article.
-
Genetic code expansion in mammalian cells: A plasmid system comparison.Bioorg Med Chem. 2020 Dec 15;28(24):115772. doi: 10.1016/j.bmc.2020.115772. Epub 2020 Sep 19. Bioorg Med Chem. 2020. PMID: 33069552 Free PMC article.
-
In-Cell Synthesis of Bioorthogonal Alkene Tag S-Allyl-Homocysteine and Its Coupling with Reprogrammed Translation.Int J Mol Sci. 2019 May 9;20(9):2299. doi: 10.3390/ijms20092299. Int J Mol Sci. 2019. PMID: 31075919 Free PMC article.
-
Engineering Pyrrolysine Systems for Genetic Code Expansion and Reprogramming.Chem Rev. 2024 Oct 9;124(19):11008-11062. doi: 10.1021/acs.chemrev.4c00243. Epub 2024 Sep 5. Chem Rev. 2024. PMID: 39235427 Free PMC article. Review.
-
The First MS-Cleavable, Photo-Thiol-Reactive Cross-Linker for Protein Structural Studies.J Am Soc Mass Spectrom. 2019 Jan;30(1):139-148. doi: 10.1007/s13361-018-1952-8. Epub 2018 Apr 20. J Am Soc Mass Spectrom. 2019. PMID: 29679287
References
-
- Stephanopoulos N, Francis MB (2011) Choosing an effective protein bioconjugation strategy. Nat Chem Biol 7: 876–884. - PubMed
-
- Tsien RY (2009) Constructing and Exploiting the Fluorescent Protein Paintbox (Nobel Lecture). Angew Chem Int Ed Engl 48: 5612–5626. - PubMed
-
- Giepmans BN, Adams SR, Ellisman MH, Tsien RY (2006) The fluorescent toolbox for assessing protein location and function. Science 312: 217–224. - PubMed
-
- Hinner MJ, Johnsson K (2010) How to obtain labeled proteins and what to do with them. Curr Opin Biotechnol 21: 766–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

