Inhibition of the MDM2 E3 Ligase induces apoptosis and autophagy in wild-type and mutant p53 models of multiple myeloma, and acts synergistically with ABT-737
- PMID: 25181509
- PMCID: PMC4151993
- DOI: 10.1371/journal.pone.0103015
Inhibition of the MDM2 E3 Ligase induces apoptosis and autophagy in wild-type and mutant p53 models of multiple myeloma, and acts synergistically with ABT-737
Abstract
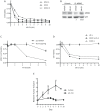
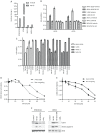
Intracellular proteolytic pathways have been validated as rational targets in multiple myeloma with the approval of two proteasome inhibitors in this disease, and with the finding that immunomodulatory agents work through an E3 ubiquitin ligase containing Cereblon. Another E3 ligase that could be a rational target is the murine double minute (MDM) 2 protein, which plays a role in p53 turnover. A novel inhibitor of this complex, MI-63, was found to induce apoptosis in p53 wild-type myeloma models in association with activation of a p53-mediated cell death program. MI-63 overcame adhesion-mediated drug resistance, showed anti-tumor activity in vivo, enhanced the activity of bortezomib and lenalidomide, and also overcame lenalidomide resistance. In mutant p53 models, inhibition of MDM2 with MI-63 also activated apoptosis, albeit at higher concentrations, and this was associated with activation of autophagy. When MI-63 was combined with the BH3 mimetic ABT-737, enhanced activity was seen in both wild-type and mutant p53 models. Finally, this regimen showed efficacy against primary plasma cells from patients with newly diagnosed and relapsed/refractory myeloma. These findings support the translation of novel MDM2 inhibitors both alone, and in combination with other novel agents, to the clinic for patients with multiple myeloma.
Conflict of interest statement
Figures



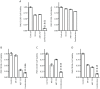
Similar articles
-
MI-63: a novel small-molecule inhibitor targets MDM2 and induces apoptosis in embryonal and alveolar rhabdomyosarcoma cells with wild-type p53.Br J Cancer. 2009 Sep 1;101(5):774-81. doi: 10.1038/sj.bjc.6605199. Br J Cancer. 2009. PMID: 19707204 Free PMC article.
-
Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML.Cell Cycle. 2006 Dec;5(23):2778-86. doi: 10.4161/cc.5.23.3520. Epub 2006 Dec 1. Cell Cycle. 2006. PMID: 17172851
-
Treatment with a BH3 mimetic overcomes the resistance of latency III EBV (+) cells to p53-mediated apoptosis.Cell Death Dis. 2011 Jul 28;2(7):e184. doi: 10.1038/cddis.2011.67. Cell Death Dis. 2011. PMID: 21796156 Free PMC article.
-
[Research Progress on the Role of Autophagy Pathway in the Pharmacological Treatment of Multiple Myeloma --Review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2024 Oct;32(5):1618-1621. doi: 10.19746/j.cnki.issn.1009-2137.2024.05.049. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2024. PMID: 39479857 Review. Chinese.
-
Autophagy: A Potential Therapeutic Target to Tackle Drug Resistance in Multiple Myeloma.Int J Mol Sci. 2023 Mar 23;24(7):6019. doi: 10.3390/ijms24076019. Int J Mol Sci. 2023. PMID: 37046991 Free PMC article. Review.
Cited by
-
Helping the Released Guardian: Drug Combinations for Supporting the Anticancer Activity of HDM2 (MDM2) Antagonists.Cancers (Basel). 2019 Jul 19;11(7):1014. doi: 10.3390/cancers11071014. Cancers (Basel). 2019. PMID: 31331108 Free PMC article. Review.
-
High cell density increases glioblastoma cell viability under glucose deprivation via degradation of the cystine/glutamate transporter xCT (SLC7A11).J Biol Chem. 2020 May 15;295(20):6936-6945. doi: 10.1074/jbc.RA119.012213. Epub 2020 Apr 7. J Biol Chem. 2020. PMID: 32265299 Free PMC article.
-
Regulatory role of E3 ubiquitin ligases in multiple myeloma: from molecular mechanisms to therapeutic strategies.Front Cell Dev Biol. 2025 Jul 30;13:1620097. doi: 10.3389/fcell.2025.1620097. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40809696 Free PMC article. Review.
-
Outcomes in patients with multiple myeloma with TP53 deletion after autologous hematopoietic stem cell transplant.Am J Hematol. 2016 Oct;91(10):E442-7. doi: 10.1002/ajh.24487. Epub 2016 Sep 3. Am J Hematol. 2016. PMID: 27420405 Free PMC article.
-
Cell Survival and Cell Death at the Intersection of Autophagy and Apoptosis: Implications for Current and Future Cancer Therapeutics.ACS Pharmacol Transl Sci. 2021 Nov 3;4(6):1728-1746. doi: 10.1021/acsptsci.1c00130. eCollection 2021 Dec 10. ACS Pharmacol Transl Sci. 2021. PMID: 34927007 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

