Metabolic engineering of microorganisms for the production of higher alcohols
- PMID: 25182323
- PMCID: PMC4173780
- DOI: 10.1128/mBio.01524-14
Metabolic engineering of microorganisms for the production of higher alcohols
Abstract
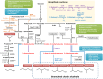
Due to the increasing concerns about limited fossil resources and environmental problems, there has been much interest in developing biofuels from renewable biomass. Ethanol is currently used as a major biofuel, as it can be easily produced by existing fermentation technology, but it is not the best biofuel due to its low energy density, high vapor pressure, hygroscopy, and incompatibility with current infrastructure. Higher alcohols, including 1-propanol, 1-butanol, isobutanol, 2-methyl-1-butanol, and 3-methyl-1-butanol, which possess fuel properties more similar to those of petroleum-based fuel, have attracted particular interest as alternatives to ethanol. Since microorganisms isolated from nature do not allow production of these alcohols at high enough efficiencies, metabolic engineering has been employed to enhance their production. Here, we review recent advances in metabolic engineering of microorganisms for the production of higher alcohols.
Copyright © 2014 Choi et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
