Enhanced in vitro formation and antibiotic resistance of nonattached Pseudomonas aeruginosa aggregates through incorporation of neutrophil products
- PMID: 25182651
- PMCID: PMC4249413
- DOI: 10.1128/AAC.03514-14
Enhanced in vitro formation and antibiotic resistance of nonattached Pseudomonas aeruginosa aggregates through incorporation of neutrophil products
Abstract
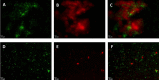
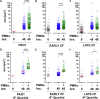
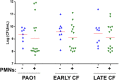
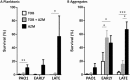
Pseudomonas aeruginosa is a major pathogen in cystic fibrosis (CF) lung disease. Children with CF are routinely exposed to P. aeruginosa from the natural environment, and by adulthood, 80% of patients are chronically infected. P. aeruginosa in the CF airway exhibits a unique biofilm-like structure, where it grows in small clusters or aggregates of bacteria in association with abundant polymers of neutrophil-derived components F-actin and DNA, among other components. These aggregates differ substantially in size and appearance compared to surface-attached in vitro biofilm models classically utilized for studies but are believed to share properties of surface-attached biofilms, including antibiotic resistance. However, little is known about the formation and function of surface-independent modes of biofilm growth, how they might be eradicated, and quorum sensing communication. To address these issues, we developed a novel in vitro model of P. aeruginosa aggregates incorporating human neutrophil-derived products. Aggregates grown in vitro and those found in CF patients' sputum samples were morphologically similar; viable bacteria were distributed in small pockets throughout the aggregate. The lasA quorum sensing gene was differentially expressed in the presence of neutrophil products. Importantly, aggregates formed in the presence of neutrophils acquired resistance to tobramycin, which was lost when the aggregates were dispersed with DNase, and antagonism of tobramycin and azithromycin was observed. This novel yet simple in vitro system advances our ability to model infection of the CF airway and will be an important tool to study virulence and test alternative eradication strategies against P. aeruginosa.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmonol. 32:356–366. 10.1002/ppul.1144. - DOI - PubMed
-
- Cystic Fibrosis Foundation. 2013. Cystic Fibrosis Foundation patient registry. 2012 annual data report to the center directors Cystic Fibrosis Foundation, Bethesda, MD.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

