Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial
- PMID: 25183726
- PMCID: PMC4188685
- DOI: 10.1503/cmaj.140495
Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial
Abstract
Background: Disturbance of the sleep-wake cycle is a characteristic of delirium. In addition, changes in melatonin rhythm influence the circadian rhythm and are associated with delirium. We compared the effect of melatonin and placebo on the incidence and duration of delirium.
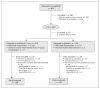
Methods: We performed this multicentre, double-blind, randomized controlled trial between November 2008 and May 2012 in 1 academic and 2 nonacademic hospitals. Patients aged 65 years or older who were scheduled for acute hip surgery were eligible for inclusion. Patients received melatonin 3 mg or placebo in the evening for 5 consecutive days, starting within 24 hours after admission. The primary outcome was incidence of delirium within 8 days of admission. We also monitored the duration of delirium.
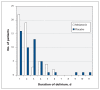
Results: A total of 452 patients were randomly assigned to the 2 study groups. We subsequently excluded 74 patients for whom the primary end point could not be measured or who had delirium before the second day of the study. After these postrandomization exclusions, data for 378 patients were included in the main analysis. The overall mean age was 84 years, 238 (63.0%) of the patients lived at home before admission, and 210 (55.6%) had cognitive impairment. We observed no effect of melatonin on the incidence of delirium: 55/186 (29.6%) in the melatonin group v. 49/192 (25.5%) in the placebo group; difference 4.1 (95% confidence interval -0.05 to 13.1) percentage points. There were no between-group differences in mortality or in cognitive or functional outcomes at 3-month follow-up.
Interpretation: In this older population with hip fracture, treatment with melatonin did not reduce the incidence of delirium.
Trial registration: Netherlands Trial Registry, NTR1576: MAPLE (Melatonin Against PLacebo in Elderly patients) study; www.trialregister.nl/trialreg/admin/rctview.asp?TC=1576.
© 2014 Canadian Medical Association or its licensors.
Figures
References
-
- Witlox J, Eurelings LS, de Jonghe JF, et al. . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443–51 - PubMed
-
- O’Mahony R, Murthy L, Akunne A, et al. . Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Intern Med 2011;154:746–51 - PubMed
-
- Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. . Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc 2005; 53:1658–66 - PubMed
-
- Ballard C, Hanney ML, Theodoulou M, et al. . The dementia anti-psychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 2009;8:151–7 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
