DAP1, a negative regulator of autophagy, controls SubAB-mediated apoptosis and autophagy
- PMID: 25183729
- PMCID: PMC4249318
- DOI: 10.1128/IAI.02213-14
DAP1, a negative regulator of autophagy, controls SubAB-mediated apoptosis and autophagy
Abstract
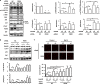
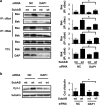
Autophagy and apoptosis play critical roles in cellular homeostasis and survival. Subtilase cytotoxin (SubAB), produced by non-O157 type Shiga-toxigenic Escherichia coli (STEC), is an important virulence factor in disease. SubAB, a protease, cleaves a specific site on the endoplasmic reticulum (ER) chaperone protein BiP/GRP78, leading to ER stress, and induces apoptosis. Here we report that in HeLa cells, activation of a PERK (RNA-dependent protein kinase [PKR]-like ER kinase)-eIF2α (α subunit of eukaryotic initiation factor 2)-dependent pathway by SubAB-mediated BiP cleavage negatively regulates autophagy and induces apoptosis through death-associated protein 1 (DAP1). We found that SubAB treatment decreased the amounts of autophagy markers LC3-II and p62 as well as those of mTOR (mammalian target of rapamycin) signaling proteins ULK1 and S6K. These proteins showed increased expression levels in PERK knockdown or DAP1 knockdown cells. In addition, depletion of DAP1 in HeLa cells dramatically inhibited the SubAB-stimulated apoptotic pathway: SubAB-induced Bax/Bak conformational changes, Bax/Bak oligomerization, cytochrome c release, activation of caspases, and poly(ADP-ribose) polymerase (PARP) cleavage. These results show that DAP1 is a key regulator, through PERK-eIF2α-dependent pathways, of the induction of apoptosis and reduction of autophagy by SubAB.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Subtilase cytotoxin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli induces stress granule formation.Cell Microbiol. 2016 Jul;18(7):1024-40. doi: 10.1111/cmi.12565. Epub 2016 Feb 21. Cell Microbiol. 2016. PMID: 26749168 Free PMC article.
-
Regulation of subtilase cytotoxin-induced cell death by an RNA-dependent protein kinase-like endoplasmic reticulum kinase-dependent proteasome pathway in HeLa cells.Infect Immun. 2012 May;80(5):1803-14. doi: 10.1128/IAI.06164-11. Epub 2012 Feb 21. Infect Immun. 2012. PMID: 22354021 Free PMC article.
-
Subtilase cytotoxin induces apoptosis in HeLa cells by mitochondrial permeabilization via activation of Bax/Bak, independent of C/EBF-homologue protein (CHOP), Ire1alpha or JNK signaling.Microb Pathog. 2010 Oct;49(4):153-63. doi: 10.1016/j.micpath.2010.05.007. Epub 2010 Jun 2. Microb Pathog. 2010. PMID: 20561923 Free PMC article.
-
Host response to the subtilase cytotoxin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli.Microbiol Immunol. 2020 Oct;64(10):657-665. doi: 10.1111/1348-0421.12841. Epub 2020 Sep 29. Microbiol Immunol. 2020. PMID: 32902863 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Identification of autophagy-associated circRNAs in sepsis-induced cardiomyopathy of mice.Sci Rep. 2023 Jul 21;13(1):11807. doi: 10.1038/s41598-023-38998-7. Sci Rep. 2023. PMID: 37479790 Free PMC article.
-
Divalent Metal Transporter 1 Knock-Down Modulates IL-1β Mediated Pancreatic Beta-Cell Pro-Apoptotic Signaling Pathways through the Autophagic Machinery.Int J Mol Sci. 2021 Jul 27;22(15):8013. doi: 10.3390/ijms22158013. Int J Mol Sci. 2021. PMID: 34360779 Free PMC article.
-
Toxins of Locus of Enterocyte Effacement-Negative Shiga Toxin-Producing Escherichia coli.Toxins (Basel). 2018 Jun 14;10(6):241. doi: 10.3390/toxins10060241. Toxins (Basel). 2018. PMID: 29903982 Free PMC article. Review.
-
Heterogeneous Family of Cyclomodulins: Smart Weapons That Allow Bacteria to Hijack the Eukaryotic Cell Cycle and Promote Infections.Front Cell Infect Microbiol. 2017 May 23;7:208. doi: 10.3389/fcimb.2017.00208. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28589102 Free PMC article. Review.
-
Cytoplasmic vacuolization in cell death and survival.Oncotarget. 2016 Aug 23;7(34):55863-55889. doi: 10.18632/oncotarget.10150. Oncotarget. 2016. PMID: 27331412 Free PMC article. Review.
References
-
- Shiomi M, Togawa M. 1997. Sporadic cases of hemolytic uremic syndrome and hemorrhagic colitis with serum IgM antibodies to lipopolysaccharides of enterohemorrhagic Escherichia coli O157. Nihon Rinsho 55:686–692 (In Japanese.) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

