Rat indwelling urinary catheter model of Candida albicans biofilm infection
- PMID: 25183731
- PMCID: PMC4249295
- DOI: 10.1128/IAI.02284-14
Rat indwelling urinary catheter model of Candida albicans biofilm infection
Abstract
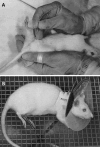
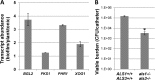
Indwelling urinary catheters are commonly used in the management of hospitalized patients. Candida can adhere to the device surface and propagate as a biofilm. These Candida biofilm communities differ from free-floating Candida, exhibiting high tolerance to antifungal therapy. The significance of catheter-associated candiduria is often unclear, and treatment may be problematic considering the biofilm drug-resistant phenotype. Here we describe a rodent model for the study of urinary catheter-associated Candida albicans biofilm infection that mimics this common process in patients. In the setting of a functioning, indwelling urinary catheter in a rat, Candida proliferated as a biofilm on the device surface. Characteristic biofilm architecture was observed, including adherent, filamentous cells embedded in an extracellular matrix. Similar to what occurs in human patients, animals with this infection developed candiduria and pyuria. Infection progressed to cystitis, and a biofilmlike covering was observed over the bladder surface. Furthermore, large numbers of C. albicans cells were dispersed into the urine from either the catheter or bladder wall biofilm over the infection period. We successfully utilized the model to test the efficacy of antifungals, analyze transcriptional patterns, and examine the phenotype of a genetic mutant. The model should be useful for future investigations involving the pathogenesis, diagnosis, therapy, prevention, and drug resistance of Candida biofilms in the urinary tract.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Plasticity of Candida albicans Biofilms.Microbiol Mol Biol Rev. 2016 Jun 1;80(3):565-95. doi: 10.1128/MMBR.00068-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27250770 Free PMC article. Review.
-
Antifungal activity of a β-peptide in synthetic urine media: Toward materials-based approaches to reducing catheter-associated urinary tract fungal infections.Acta Biomater. 2016 Oct 1;43:240-250. doi: 10.1016/j.actbio.2016.07.016. Epub 2016 Jul 12. Acta Biomater. 2016. PMID: 27422198 Free PMC article.
-
ELECTRON MICROSCOPIC ASSAY OF BACTERIAL BIOFILM FORMED ON INDWELLING URETHRAL CATHETERS.J Egypt Soc Parasitol. 2016 Dec;46(3):475-484. J Egypt Soc Parasitol. 2016. PMID: 30230743
-
Host contributions to construction of three device-associated Candida albicans biofilms.Infect Immun. 2015 Dec;83(12):4630-8. doi: 10.1128/IAI.00931-15. Epub 2015 Sep 14. Infect Immun. 2015. PMID: 26371129 Free PMC article.
-
Bacterial biofilm formation on indwelling urethral catheters.Lett Appl Microbiol. 2019 Apr;68(4):277-293. doi: 10.1111/lam.13144. Lett Appl Microbiol. 2019. PMID: 30811615 Review.
Cited by
-
Assessment of Antifungal Pharmacodynamics.J Fungi (Basel). 2023 Feb 1;9(2):192. doi: 10.3390/jof9020192. J Fungi (Basel). 2023. PMID: 36836307 Free PMC article.
-
How Biofilm Growth Affects Candida-Host Interactions.Front Microbiol. 2020 Jun 25;11:1437. doi: 10.3389/fmicb.2020.01437. eCollection 2020. Front Microbiol. 2020. PMID: 32670252 Free PMC article. Review.
-
Management of candiduria in hospitalized patients: a single-center study on the implementation of IDSA guidelines and factors affecting clinical decisions.Eur J Clin Microbiol Infect Dis. 2021 Jan;40(1):59-65. doi: 10.1007/s10096-020-03999-1. Epub 2020 Jul 30. Eur J Clin Microbiol Infect Dis. 2021. PMID: 32734337
-
Fungal Biofilms and Polymicrobial Diseases.J Fungi (Basel). 2017 May 10;3(2):22. doi: 10.3390/jof3020022. J Fungi (Basel). 2017. PMID: 29371540 Free PMC article. Review.
-
Plasticity of Candida albicans Biofilms.Microbiol Mol Biol Rev. 2016 Jun 1;80(3):565-95. doi: 10.1128/MMBR.00068-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27250770 Free PMC article. Review.
References
-
- Bouza E, San Juan R, Munoz P, Voss A, Kluytmans J, Co-operative Group of the European Study Group on Nosocomial Infections 2001. A European perspective on nosocomial urinary tract infections I. Report on the microbiology workload, etiology and antimicrobial susceptibility (ESGNI-003 study). European Study Group on Nosocomial Infections. Clin. Microbiol. Infect. 7:523–531. 10.1046/j.1198-743x.2001.00326.x. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

