The neomuran revolution and phagotrophic origin of eukaryotes and cilia in the light of intracellular coevolution and a revised tree of life
- PMID: 25183828
- PMCID: PMC4142966
- DOI: 10.1101/cshperspect.a016006
The neomuran revolution and phagotrophic origin of eukaryotes and cilia in the light of intracellular coevolution and a revised tree of life
Abstract
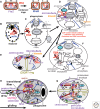
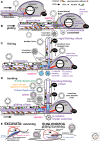
Three kinds of cells exist with increasingly complex membrane-protein targeting: Unibacteria (Archaebacteria, Posibacteria) with one cytoplasmic membrane (CM); Negibacteria with a two-membrane envelope (inner CM; outer membrane [OM]); eukaryotes with a plasma membrane and topologically distinct endomembranes and peroxisomes. I combine evidence from multigene trees, palaeontology, and cell biology to show that eukaryotes and archaebacteria are sisters, forming the clade neomura that evolved ~1.2 Gy ago from a posibacterium, whose DNA segregation and cell division were destabilized by murein wall loss and rescued by the evolving novel neomuran endoskeleton, histones, cytokinesis, and glycoproteins. Phagotrophy then induced coevolving serial major changes making eukaryote cells, culminating in two dissimilar cilia via a novel gliding-fishing-swimming scenario. I transfer Chloroflexi to Posibacteria, root the universal tree between them and Heliobacteria, and argue that Negibacteria are a clade whose OM, evolving in a green posibacterium, was never lost.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Adams DW, Errington J 2009. Bacterial cell division: Assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7: 642–653 - PubMed
-
- Bapteste E, Susko E, Leigh J, Ruiz-Trillo I, Bucknam J, Doolittle WF 2008. Alternative methods for concatenation of core genes indicate a lack of resolution in deep nodes of the prokaryotic phylogeny. Mol Biol Evol 25: 83–91 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
