Epigenetic regulation in plant responses to the environment
- PMID: 25183832
- PMCID: PMC4142964
- DOI: 10.1101/cshperspect.a019471
Epigenetic regulation in plant responses to the environment
Abstract
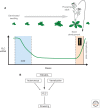
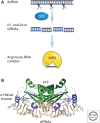
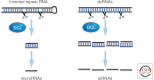
In this article, we review environmentally mediated epigenetic regulation in plants using two case histories. One of these, vernalization, mediates adaptation of plants to different environments and it exemplifies processes that are reset in each generation. The other, virus-induced silencing, involves transgenerationally inherited epigenetic modifications. Heritable epigenetic marks may result in heritable phenotypic variation, influencing fitness, and so be subject to natural selection. However, unlike genetic inheritance, the epigenetic modifications show instability and are influenced by the environment. These two case histories are then compared with other phenomena in plant biology that are likely to represent epigenetic regulation in response to the environment.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


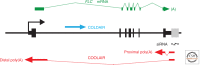




References
-
- Angel A, Song J, Dean C, Howard MA 2011. A Polycomb-based switch underlying quantitative epigenetic memory. Nature 476: 105–108 - PubMed
-
- Baulcombe DC 1999. Fast forward genetics based on virus-induced gene silencing. Curr Opin Plant Biol 2: 109–113 - PubMed
-
- Baulcombe D 2004. RNA silencing in plants. Nature 431: 356–363 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
