Metformin directly acts on mitochondria to alter cellular bioenergetics
- PMID: 25184038
- PMCID: PMC4147388
- DOI: 10.1186/2049-3002-2-12
Metformin directly acts on mitochondria to alter cellular bioenergetics
Abstract
Background: Metformin is widely used in the treatment of diabetes, and there is interest in 'repurposing' the drug for cancer prevention or treatment. However, the mechanism underlying the metabolic effects of metformin remains poorly understood.
Methods: We performed respirometry and stable isotope tracer analyses on cells and isolated mitochondria to investigate the impact of metformin on mitochondrial functions.
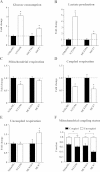
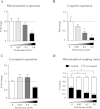
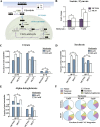
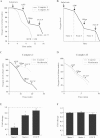
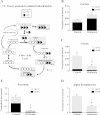
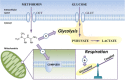
Results: We show that metformin decreases mitochondrial respiration, causing an increase in the fraction of mitochondrial respiration devoted to uncoupling reactions. Thus, cells treated with metformin become energetically inefficient, and display increased aerobic glycolysis and reduced glucose metabolism through the citric acid cycle. Conflicting prior studies proposed mitochondrial complex I or various cytosolic targets for metformin action, but we show that the compound limits respiration and citric acid cycle activity in isolated mitochondria, indicating that at least for these effects, the mitochondrion is the primary target. Finally, we demonstrate that cancer cells exposed to metformin display a greater compensatory increase in aerobic glycolysis than nontransformed cells, highlighting their metabolic vulnerability. Prevention of this compensatory metabolic event in cancer cells significantly impairs survival.
Conclusions: Together, these results demonstrate that metformin directly acts on mitochondria to limit respiration and that the sensitivity of cells to metformin is dependent on their ability to cope with energetic stress.
Keywords: Cancer; Citric acid cycle; Complex I; Metabolism; Metformin; Mitochondria; Respiration.
Figures

Comment in
-
Understanding the complex-I-ty of metformin action: limiting mitochondrial respiration to improve cancer therapy.BMC Biol. 2014 Oct 24;12:82. doi: 10.1186/s12915-014-0082-4. BMC Biol. 2014. PMID: 25347702 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

