Multicenter study of trimethoprim/sulfamethoxazole-related hepatotoxicity: incidence and associated factors among HIV-infected patients treated for Pneumocystis jirovecii pneumonia
- PMID: 25184238
- PMCID: PMC4153565
- DOI: 10.1371/journal.pone.0106141
Multicenter study of trimethoprim/sulfamethoxazole-related hepatotoxicity: incidence and associated factors among HIV-infected patients treated for Pneumocystis jirovecii pneumonia
Abstract
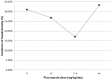
The incidence of hepatotoxicity related to trimethoprim/sulfamethoxazole (TMP/SMX) administered at a therapeutic dose may vary among study populations of different ethnicities and hepatotoxic metabolites of TMP/SMX may be decreased by drug-drug interaction with fluconazole. We aimed to investigate the incidence of hepatotoxicity and the role of concomitant use of fluconazole in HIV-infected patients receiving TMP/SMX for Pneumocystis jirovecii pneumonia. We reviewed medical records to collect clinical characteristics and laboratory data of HIV-infected patients who received TMP/SMX for treatment of P. jirovecii pneumonia at 6 hospitals around Taiwan between September 2009 and February 2013. Hepatotoxicity was defined as 2-fold or greater increase of aminotransferase or total bilirubin level from baselines. Roussel UCLAF Causality Assessment Method (RUCAM) was used to analyze the causality of drug-induced liver injuries. NAT1 and NAT2 acetylator types were determined with the use of polymerase-chain-reaction (PCR) restriction fragment length polymorphism to differentiate common single-nucleotide polymorphisms (SNPs) predictive of the acetylator phenotypes in a subgroup of patients. During the study period, 286 courses of TMP/SMX treatment administered to 284 patients were analyzed. One hundred and fifty-two patients (53.1%) developed hepatotoxicity, and TMP/SMX was considered causative in 47 (16.4%) who had a RUCAM score of 6 or greater. In multivariate analysis, concomitant use of fluconazole for candidiasis was the only factor associated with reduced risk for hepatotoxicity (adjusted odds ratio, 0.372; 95% confidence interval, 0.145-0.957), while serostatus of hepatitis B or C virus, NAT1 and NAT2 acetylator types, or receipt of combination antiretroviral therapy was not. The incidence of hepatotoxicity decreased with an increasing daily dose of fluconazole up to 4.0 mg/kg. We conclude that the incidence of TMP/SMX-related hepatotoxicity was 16.4% in HIV-infected Taiwanese patients who received TMP/SMX for pneumocystosis. Concomitant use of fluconazole was associated with decreased risk for TMP/SMX-related hepatotoxicity.
Conflict of interest statement
Figures

References
-
- Lee KY, Ho CC, Ji DD, Lee CM, Tsai MS, et al. (2012) Etiology of pulmonary complications of human immunodeficiency virus-1-infected patients in Taiwan in the era of combination antiretroviral therapy: A prospective observational study. J Microbiol Immunol Infect - PubMed
-
- Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, et al. (2000) Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 30 Suppl 1: S5–14. - PubMed
-
- Miller RF, Huang L, Walzer PD (2013) Pneumocystis pneumonia associated with human immunodeficiency virus. Clin Chest Med 34: 229–241. - PubMed
-
- Lawson DH, Paice BJ (1982) Adverse reactions to trimethoprim-sulfamethoxazole. Rev Infect Dis 4: 429–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

