From blue light to clock genes in zebrafish ZEM-2S cells
- PMID: 25184495
- PMCID: PMC4153568
- DOI: 10.1371/journal.pone.0106252
From blue light to clock genes in zebrafish ZEM-2S cells
Abstract
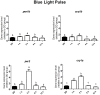
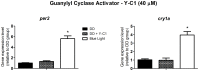
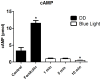
Melanopsin has been implicated in the mammalian photoentrainment by blue light. This photopigment, which maximally absorbs light at wavelengths between 470 and 480 nm depending on the species, is found in the retina of all classes of vertebrates so far studied. In mammals, melanopsin activation triggers a signaling pathway which resets the circadian clock in the suprachiasmatic nucleus (SCN). Unlike mammals, Drosophila melanogaster and Danio rerio do not rely only on their eyes to perceive light, in fact their whole body may be capable of detecting light and entraining their circadian clock. Melanopsin, teleost multiple tissue (tmt) opsin and others such as neuropsin and va-opsin, are found in the peripheral tissues of Danio rerio, however, there are limited data concerning the photopigment/s or the signaling pathway/s directly involved in light detection. Here, we demonstrate that melanopsin is a strong candidate to mediate synchronization of zebrafish cells. The deduced amino acid sequence of melanopsin, although being a vertebrate opsin, is more similar to invertebrate than vertebrate photopigments, and melanopsin photostimulation triggers the phosphoinositide pathway through activation of a G(q/11)-type G protein. We stimulated cultured ZEM-2S cells with blue light at wavelengths consistent with melanopsin maximal absorption, and evaluated the time course expression of per1b, cry1b, per2 and cry1a. Using quantitative PCR, we showed that blue light is capable of slightly modulating per1b and cry1b genes, and drastically increasing per2 and cry1a expression. Pharmacological assays indicated that per2 and cry1a responses to blue light are evoked through the activation of the phosphoinositide pathway, which crosstalks with nitric oxide (NO) and mitogen activated protein MAP kinase (MAPK) to activate the clock genes. Our results suggest that melanopsin may be important in mediating the photoresponse in Danio rerio ZEM-2S cells, and provide new insights about the modulation of clock genes in peripheral clocks.
Conflict of interest statement
Figures







Similar articles
-
Melanopsin and clock genes: regulation by light and endothelin in the zebrafish ZEM-2S cell line.Chronobiol Int. 2009 Aug;26(6):1090-119. doi: 10.3109/07420520903249005. Chronobiol Int. 2009. PMID: 19731108
-
Light induction of a vertebrate clock gene involves signaling through blue-light receptors and MAP kinases.Curr Biol. 2002 May 14;12(10):844-8. doi: 10.1016/s0960-9822(02)00835-7. Curr Biol. 2002. PMID: 12015122
-
Melanopsin--shedding light on the elusive circadian photopigment.Chronobiol Int. 2004 Mar;21(2):189-204. doi: 10.1081/cbi-120037816. Chronobiol Int. 2004. PMID: 15332341 Free PMC article. Review.
-
Dexamethasone Modulates Nonvisual Opsins, Glucocorticoid Receptor, and Clock Genes in Danio rerio ZEM-2S Cells.Biomed Res Int. 2017;2017:8459385. doi: 10.1155/2017/8459385. Epub 2017 May 14. Biomed Res Int. 2017. PMID: 28589149 Free PMC article.
-
Melanopsin: a novel photopigment involved in the photoentrainment of the brain's biological clock?Ann Med. 2002;34(5):401-7. doi: 10.1080/078538902320772151. Ann Med. 2002. PMID: 12452484 Review.
Cited by
-
Cannabinoid Receptor 1 Regulates Zebrafish Renal Multiciliated Cell Development via cAMP Signaling.J Dev Biol. 2025 Jun 17;13(2):20. doi: 10.3390/jdb13020020. J Dev Biol. 2025. PMID: 40558673 Free PMC article.
-
Heat-inactivated Streptococcus pneumoniae augments circadian clock gene expression in zebrafish cells.Sci Rep. 2024 Nov 13;14(1):27805. doi: 10.1038/s41598-024-78888-0. Sci Rep. 2024. PMID: 39537820 Free PMC article.
-
The Light Wavelength Affects the Ontogeny of Clock Gene Expression and Activity Rhythms in Zebrafish Larvae.PLoS One. 2015 Jul 6;10(7):e0132235. doi: 10.1371/journal.pone.0132235. eCollection 2015. PLoS One. 2015. PMID: 26147202 Free PMC article.
-
Opsins outside the eye and the skin: a more complex scenario than originally thought for a classical light sensor.Cell Tissue Res. 2021 Sep;385(3):519-538. doi: 10.1007/s00441-021-03500-0. Epub 2021 Jul 8. Cell Tissue Res. 2021. PMID: 34236517 Review.
-
Screening Algal and Cyanobacterial Extracts to Identify Potential Substitutes for Fetal Bovine Serum in Cellular Meat Cultivation.Foods. 2024 Nov 22;13(23):3741. doi: 10.3390/foods13233741. Foods. 2024. PMID: 39682813 Free PMC article.
References
-
- Jenkins A, Munoz M, Tarttelin EE, Bellingham J, Foster RG, et al. (2003) VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr Biol 13: 1269–1278. - PubMed
-
- Bailey MJ, Cassone VM (2005) Melanopsin expression in the chick retina and pineal gland. Brain Res Mol Brain Res 134: 345–348. - PubMed
-
- Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, et al. (2005) Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem 92: 158–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

