Picornavirus morphogenesis
- PMID: 25184560
- PMCID: PMC4187686
- DOI: 10.1128/MMBR.00012-14
Picornavirus morphogenesis
Abstract
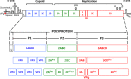
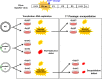
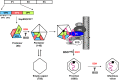
The Picornaviridae represent a large family of small plus-strand RNA viruses that cause a bewildering array of important human and animal diseases. Morphogenesis is the least-understood step in the life cycle of these viruses, and this process is difficult to study because encapsidation is tightly coupled to genome translation and RNA replication. Although the basic steps of assembly have been known for some time, very few details are available about the mechanism and factors that regulate this process. Most of the information available has been derived from studies of enteroviruses, in particular poliovirus, where recent evidence has shown that, surprisingly, the specificity of encapsidation is governed by a viral protein-protein interaction that does not involve an RNA packaging signal. In this review, we make an attempt to summarize what is currently known about the following topics: (i) encapsidation intermediates, (ii) the specificity of encapsidation (iii), viral and cellular factors that are required for encapsidation, (iv) inhibitors of encapsidation, and (v) a model of enterovirus encapsidation. Finally, we compare some features of picornavirus morphogenesis with those of other plus-strand RNA viruses.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Knowles E, Delwart E, Gorbalenya AE, Hovi T, Hyypia T, King AMQ, LinBerg AM, Pallansch MA, Palmenberg AC, Reuter G, Simmonds P, Skern T, Stanway G, Yamashita T, Zell R. 2014. Picornaviridae: 26 genera, 46 species and growing, p 98 Abstr. Europic 18th Int. Picornavirus Meet., Blankenberge, Belgium
-
- Stuart DWX, Ren J, Gao Q, Hu Z, Sun Y, Li X, Rowlands D, Yin W, Wang J, Rao Y, Fry E. 2014. Structural analysis of Hepatitis A virus, p P30 Abstr. Europic 18th Int. Picornavirus Meet., Blankenberge, Belgium
-
- Fry EE, Stuart DI. 2010. Virion structure, p 59–72 In Ehrenfeld E, Domingo E, Roos RP. (ed), The picornaviruses. ASM Press, Washington, DC
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

