The Plasmodium bottleneck: malaria parasite losses in the mosquito vector
- PMID: 25185005
- PMCID: PMC4156458
- DOI: 10.1590/0074-0276130597
The Plasmodium bottleneck: malaria parasite losses in the mosquito vector
Abstract
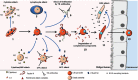
Nearly one million people are killed every year by the malaria parasite Plasmodium. Although the disease-causing forms of the parasite exist only in the human blood, mosquitoes of the genus Anopheles are the obligate vector for transmission. Here, we review the parasite life cycle in the vector and highlight the human and mosquito contributions that limit malaria parasite development in the mosquito host. We address parasite killing in its mosquito host and bottlenecks in parasite numbers that might guide intervention strategies to prevent transmission.
Figures


References
-
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

