Structure and protein-protein interactions of methanol dehydrogenase from Methylococcus capsulatus (Bath)
- PMID: 25185034
- PMCID: PMC4188263
- DOI: 10.1021/bi500850j
Structure and protein-protein interactions of methanol dehydrogenase from Methylococcus capsulatus (Bath)
Abstract
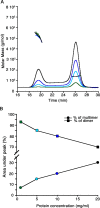
In the initial steps of their metabolic pathway, methanotrophic bacteria oxidize methane to methanol with methane monooxygenases (MMOs) and methanol to formaldehyde with methanol dehydrogenases (MDHs). Several lines of evidence suggest that the membrane-bound or particulate MMO (pMMO) and MDH interact to form a metabolic supercomplex. To further investigate the possible existence of such a supercomplex, native MDH from Methylococcus capsulatus (Bath) has been purified and characterized by size exclusion chromatography with multi-angle light scattering and X-ray crystallography. M. capsulatus (Bath) MDH is primarily a dimer in solution, although an oligomeric species with a molecular mass of ∼450-560 kDa forms at higher protein concentrations. The 2.57 Å resolution crystal structure reveals an overall fold and α2β2 dimeric architecture similar to those of other MDH structures. In addition, biolayer interferometry studies demonstrate specific protein-protein interactions between MDH and M. capsulatus (Bath) pMMO as well as between MDH and the truncated recombinant periplasmic domains of M. capsulatus (Bath) pMMO (spmoB). These interactions exhibit KD values of 833 ± 409 nM and 9.0 ± 7.7 μM, respectively. The biochemical data combined with analysis of the crystal lattice interactions observed in the MDH structure suggest a model in which MDH and pMMO associate not as a discrete, stoichiometric complex but as a larger assembly scaffolded by the intracytoplasmic membranes.
Figures





References
-
- Semrau J. D.; Dispirito A. A.; Yoon S. (2010) Methanotrophs and copper. FEMS Microbiol. Lett. 34, 496–531. - PubMed
-
- Fei Q.; Guarnieri M. T.; Tao L.; Laurens L. M.; Dowe N.; Pienkos P. T. (2014) Bioconversion of natural gas to liquid fuel: Opportunities and challenges. Biotechnol. Adv. 32, 596–614. - PubMed
-
- Hakemian A. S.; Rosenzweig A. C. (2007) The biochemistry of methane oxidation. Annu. Rev. Biochem. 76, 223–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

