Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma
- PMID: 25185097
- PMCID: PMC4588714
- DOI: 10.1200/JCO.2013.54.3660
Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma
Abstract
Purpose: TH-302, a prodrug of the cytotoxic alkylating agent bromo-isophosphoramide mustard, is preferentially activated in hypoxic conditions. This phase II study investigated TH-302 in combination with doxorubicin, followed by single-agent TH-302 maintenance therapy in patients with first-line advanced soft tissue sarcoma (STS) to assess progression-free survival (PFS), response rate, overall survival, safety, and tolerability.
Patients and methods: In this open-label phase II study, TH-302 300 mg/m(2) was administered intravenously on days 1 and 8 with doxorubicin 75 mg/m(2) on day 1 of each 21-day cycle. After six cycles, patients with stable and/or responding disease could receive maintenance monotherapy with TH-302.
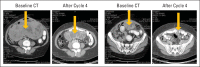
Results: Ninety-one patients initiated TH-302 plus doxorubicin induction treatment. The PFS rate at 6 months (primary efficacy measure) was 58% (95% CI, 46% to 68%). Median PFS was 6.5 months (95% CI, 5.8 to 7.7 months); median overall survival was 21.5 months (95% CI, 16.0 to 26.2 months). Best tumor responses were complete response (n = 2 [2%]) and partial response (n = 30 [34%]). During TH-302 maintenance (n = 48), five patients improved from stable disease to partial response, and one patient improved from partial to complete response. The most common adverse events during induction were fatigue, nausea, and skin and/or mucosal toxicities as well as anemia, thrombocytopenia, and neutropenia. These were less severe and less frequent during maintenance. There was no evidence of TH-302-related hepatic, renal, or cardiac toxicity.
Conclusion: PFS, overall survival, and tumor response compared favorably with historical outcomes achieved with other first-line chemotherapies for advanced STS. A phase III study of TH-302 is ongoing (NCT01440088).
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Eriksson M. Histology-driven chemotherapy of soft-tissue sarcoma. Ann Oncol. 2010;21:vii270–vii276. - PubMed
-
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. - PubMed
-
- Karavasilis V, Seddon BM, Ashley S, et al. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: Retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112:1585–1591. - PubMed
-
- Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) Eur J Cancer. 2010;46:72–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

