Role of peroxisome proliferator-activated receptor-γ in vascular muscle in the cerebral circulation
- PMID: 25185134
- PMCID: PMC4192060
- DOI: 10.1161/HYPERTENSIONAHA.114.03935
Role of peroxisome proliferator-activated receptor-γ in vascular muscle in the cerebral circulation
Abstract
Although peroxisome proliferator-activated receptor-γ (PPARγ) is thought to play a protective role in the vasculature, its cell-specific effect, particularly in resistance vessels, is poorly defined. Nitric oxide (NO) plays a major role in vascular biology in the brain. We examined the hypothesis that selective interference with PPARγ in vascular muscle would impair NO-dependent responses and augment vasoconstrictor responses in the cerebral circulation. We studied mice expressing a dominant negative mutation in human PPARγ (P467L) under the control of the smooth muscle myosin heavy chain promoter (S-P467L). In S-P467L mice, dilator responses to exogenously applied or endogenously produced NO were greatly impaired in cerebral arteries in vitro and in small cerebral arterioles in vivo. Select NO-independent responses, including vasodilation to low concentrations of potassium, were also impaired in S-P467L mice. In contrast, increased expression of wild-type PPARγ in smooth muscle had little effect on vasomotor responses. Mechanisms underlying impairment of both NO-dependent and NO-independent vasodilator responses after interference with PPARγ involved Rho kinase with no apparent contribution by oxidative stress-related mechanisms. These findings support the concept that via effects on Rho kinase-dependent signaling, PPARγ in vascular muscle is a major determinant of vascular tone in resistance vessels and, in particular, NO-mediated signaling in cerebral arteries and brain microvessels. Considering the importance of NO and Rho kinase, these findings have implications for regulation of cerebral blood flow and the pathogenesis of large and small vessel disease in brain.
Keywords: cerebral arteries; microcirculation; nitric oxide; small vessel disease.
© 2014 American Heart Association, Inc.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
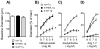
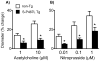



References
-
- Marchesi C, Schiffrin EL. Peroxisome proliferator-activated receptors and the vascular system: beyond their metabolic effects. J Am Soc Hypertens. 2008;2:227–238. - PubMed
-
- Plutzky J. The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ Res. 2011;108:1002–1016. - PubMed
-
- Davidson M, Meyer PM, Haffner S, Feinstein S, D’Agostino R, Sr, Kondos GT, Perez A, Chen Z, Mazzone T. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 2008;117:2123–2130. - PubMed
-
- Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90:269–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL048058/HL/NHLBI NIH HHS/United States
- NS-24621/NS/NINDS NIH HHS/United States
- HL-61446/HL/NHLBI NIH HHS/United States
- R01 HL061446/HL/NHLBI NIH HHS/United States
- HL-113863/HL/NHLBI NIH HHS/United States
- P01 HL084207/HL/NHLBI NIH HHS/United States
- HL-62984/HL/NHLBI NIH HHS/United States
- R01 HL113863/HL/NHLBI NIH HHS/United States
- P01 NS024621/NS/NINDS NIH HHS/United States
- P01 HL062984/HL/NHLBI NIH HHS/United States
- I01 BX001399/BX/BLRD VA/United States
- HL-48058/HL/NHLBI NIH HHS/United States
- R01 HL048058/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

