Impact of minimal inhibitory concentration breakpoints on local cumulative bacterial susceptibility data and antibiotic consumption
- PMID: 25185565
- PMCID: PMC4161835
- DOI: 10.1186/1756-0500-7-603
Impact of minimal inhibitory concentration breakpoints on local cumulative bacterial susceptibility data and antibiotic consumption
Abstract
Background: The phenotypic antimicrobial susceptibility testing (AST) of bacteria depends on minimal inhibitory concentration breakpoints issued by national and international breakpoint committees. The current study was performed in order to test the influence of different AST standards on local cumulative AST data and on antibiotic consumption.
Methods: Automated AST was performed with clinical isolates of Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and E. faecium. From each species 100 prospectively collected non-duplicate clinical isolates were tested and MIC data were interpreted according to the interpretation standards issued by DIN and EUCAST, respectively. In addition cumulative AST data from clinical isolates and antibiotic consumption were monitored before and after implementation of new EUCAST MIC breakpoints.
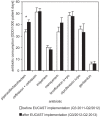
Results: The susceptibility rate of P. aeruginosa against piperacillin and gentamicin, and of C. freundii against piperacillin/tazobactam increased significantly, whereas the susceptibility rates of E. cloacae, S. marcescens, and M. morganii against ciprofloxacin decreased significantly after switching from DIN to EUCAST MIC breakpoints. These changes in the cumulative antibiotic resistance pattern were reflected by enhanced consumption of piperacillin/tazobactam after implementation of EUCAST MIC breakpoints.
Conclusions: These data show that changes of AST breakpoints have a significant influence on local cumulative AST data and on antibiotic consumption.
Figures
References
-
- Normensausschuss Medizin im DIN . Teil 4: Bewertungsstufen für die Minimale Hemmkonzentration; MHK-Grenzwerte von Antibakteriellen Wirkstoffen. In: Normung DDIf., editor. Medizinische Mikrobiologie- Empfindlichkeitsprüfung von Mikrobiellen Krankheitserregern Gegen Chemotherapeutika. Berlin, Germany: Beuth Verlag GmbH; 2004.
-
- Breakpoint tables for interpretation of MICs and zone diameters, version 2.0http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_t...
-
- Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. M100-S20. Wayne, PA, USA: CLSI; 2010.
-
- Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. - DOI - PubMed
-
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

