Distribution across tissue layers of extrinsic nerves innervating the mouse colorectum - an in vitro anterograde tracing study
- PMID: 25185752
- PMCID: PMC4200533
- DOI: 10.1111/nmo.12419
Distribution across tissue layers of extrinsic nerves innervating the mouse colorectum - an in vitro anterograde tracing study
Abstract
Background: Anterograde in vitro tracing of the pelvic nerve (PN) and visualization in the horizontal plane in whole mount preparations has been fundamental in the analysis of distribution of peripheral nerves innervating the colorectum. Here, we performed a similar analysis, but in cryostat sections of the mouse colorectum, allowing for a more direct visualization of nerve distribution in all tissue layers.
Methods: Colorectum with attached PNs was dissected from adult male BalbC mice. Presence of active afferents was certified by single fiber recording of fine PN fibers. This was followed by 'bulk' (all fibers) anterograde tracing using biotinamide (BTA). Histo- and immunohistochemical techniques were used for visualization of BTA-positive nerves, and evaluation of co-localization with calcitonin gene-related peptide (CGRP), respectively. Tissue was analyzed using confocal microscopy on transverse or longitudinal colorectum sections.
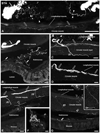
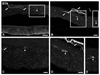
Key results: Abundant BTA-positive nerves spanning all layers of the mouse colorectum and contacting myenteric plexus neurons, distributing within the muscle layer, penetrating deeper into the organ and contacting blood vessels, submucosal plexus neurons or even penetrating the mucosa, were regularly detected. Several traced axons co-localized CGRP, supporting their afferent nature. Finally, anterograde tracing of the PN also exposed abundant BTA-positive nerves in the major pelvic ganglion.
Conclusions & inferences: We present the patterns of innervation of extrinsic axons across layers in the mouse colorectum, including the labile mucosal layer. The proposed approach could also be useful in the analysis of associations between morphology and physiology of peripheral nerves targeting the different layers of the colorectum.
Keywords: anterograde; biotinamide; colorectum; in vitro tracing; peripheral nerves; primary afferents.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
DISCLOSURE
None of the authors have any actual or potential conflict of interest.
Figures





References
-
- Blackshaw LA, Brookes SJ, Grundy D et al. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. - PubMed
-
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. - PubMed
-
- Brookes SJ, Spencer NJ, Costa M, et al. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10:286–296. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

