In vitro synergistic action of geldanamycin- and docetaxel-containing HPMA copolymer-RGDfK conjugates against ovarian cancer
- PMID: 25185891
- PMCID: PMC4262670
- DOI: 10.1002/mabi.201400360
In vitro synergistic action of geldanamycin- and docetaxel-containing HPMA copolymer-RGDfK conjugates against ovarian cancer
Abstract
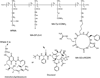
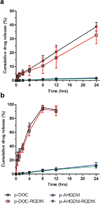
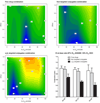
HPMA copolymer-RGDfK (HPMA-RGDfK) conjugates bearing either aminohexylgeldanamycin (AHGDM) or docetaxel (DOC) were synthesized and characterized. In vitro stability and binding were evaluated. Cytotoxicity toward ovarian cancer cells was evaluated and the ability of the conjugates to induce cell death was assessed by combination index analysis. Conjugates bearing AHGDM were more stable and exhibited slower drug release than those bearing DOC. Both conjugates demonstrated the ability to bind to avb3 integrins. In combination, HPMA-RGDfK conjugates demonstrated marked synergism as compared to their non-targeted counterparts and free drug controls. HPMA-RGDfK conjugates bearing AHGDM and DOC induce synergistic cytotoxicity in vitro, suggesting their potential as a promising combination therapy.
Keywords: docetaxel; geldanamycin; ovarian cancer; targeted drug delivery.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

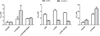
Similar articles
-
Synergistic enhancement of cancer therapy using a combination of heat shock protein targeted HPMA copolymer-drug conjugates and gold nanorod induced hyperthermia.J Control Release. 2013 Aug 28;170(1):41-50. doi: 10.1016/j.jconrel.2013.04.006. Epub 2013 Apr 17. J Control Release. 2013. PMID: 23602864 Free PMC article.
-
Biodistribution of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer drug delivery.Mol Pharm. 2009 Nov-Dec;6(6):1836-47. doi: 10.1021/mp900134c. Mol Pharm. 2009. PMID: 19743884 Free PMC article.
-
HPMA copolymer-aminohexylgeldanamycin conjugates targeting cell surface expressed GRP78 in prostate cancer.Pharm Res. 2010 Dec;27(12):2683-93. doi: 10.1007/s11095-010-0267-7. Epub 2010 Sep 16. Pharm Res. 2010. PMID: 20845065
-
HPMA copolymer delivery of chemotherapy and photodynamic therapy in ovarian cancer.Adv Exp Med Biol. 2003;519:101-23. doi: 10.1007/0-306-47932-X_7. Adv Exp Med Biol. 2003. PMID: 12675211 Review.
-
HPMA copolymer-cyclic RGD conjugates for tumor targeting.Adv Drug Deliv Rev. 2010 Feb 17;62(2):167-83. doi: 10.1016/j.addr.2009.11.027. Epub 2009 Dec 4. Adv Drug Deliv Rev. 2010. PMID: 19951733 Review.
Cited by
-
Platinum covalent shell cross-linked micelles designed to deliver doxorubicin for synergistic combination cancer therapy.Int J Nanomedicine. 2017 May 12;12:3697-3710. doi: 10.2147/IJN.S130938. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28553108 Free PMC article.
-
Polymer nanomedicines.Adv Drug Deliv Rev. 2020;156:40-64. doi: 10.1016/j.addr.2020.07.020. Epub 2020 Jul 28. Adv Drug Deliv Rev. 2020. PMID: 32735811 Free PMC article. Review.
References
-
- American Cancer Society. [assessed July 2013]; http://www.cancer.org/cancer/ovariancancer/detailedguide/ovarian-cancer-....
-
- Gonzalez-Diego P, Lopez-Abente G, Pollan M. Eur. J. Cancer. 2000;36:1816. - PubMed
-
- Rowland JH, Aziz N, Tesauro G, Feuer EJ. Semin. Oncol. Nurs. 2001;17:236. - PubMed
-
- Guarneri V, Piacentini F, Barbieri E, Conte PF. Gynecol. Oncol. 2009;117:152. - PubMed
-
- Gadducci A, Sartori E, Maggino T, Zola P, Landoni F, Fanucchi A, Palai N, Alessi C, Ferrero AM, Cosio S, Cristofani R. Gynecol. Oncol. 1998;68:150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

