Sevoflurane preconditioning ameliorates neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/reperfusion in rats
- PMID: 25186151
- PMCID: PMC4161899
- DOI: 10.1186/s13041-014-0069-7
Sevoflurane preconditioning ameliorates neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/reperfusion in rats
Abstract
Background: Microglia are the primary immune cells of the spinal cord that are activated in response to ischemia/reperfusion (IR) injury and release various neurotrophic and/or neurotoxic factors to determine neuronal survival. Among them, matrix metalloproteinase-9 (MMP-9), which cleaves various components of the extracellular matrix in the basal lamina and functions as part of the blood spinal cord barrier (BSCB), is considered important for regulating inflammatory responses and microenvironmental homeostasis of the BSCB in the pathology of ischemia. Sevoflurane has been reported to protect against neuronal apoptosis during cerebral IR. However, the effects of sevoflurane preconditioning on spinal cord IR injury remain unclear. In this study, we investigated the role of sevoflurane on potential genetic roles of microglial MMP-9 in tight junction protein breakdown, opening of the BSCB, and subsequent recruitment of microglia to apoptotic spinal cord neurons.
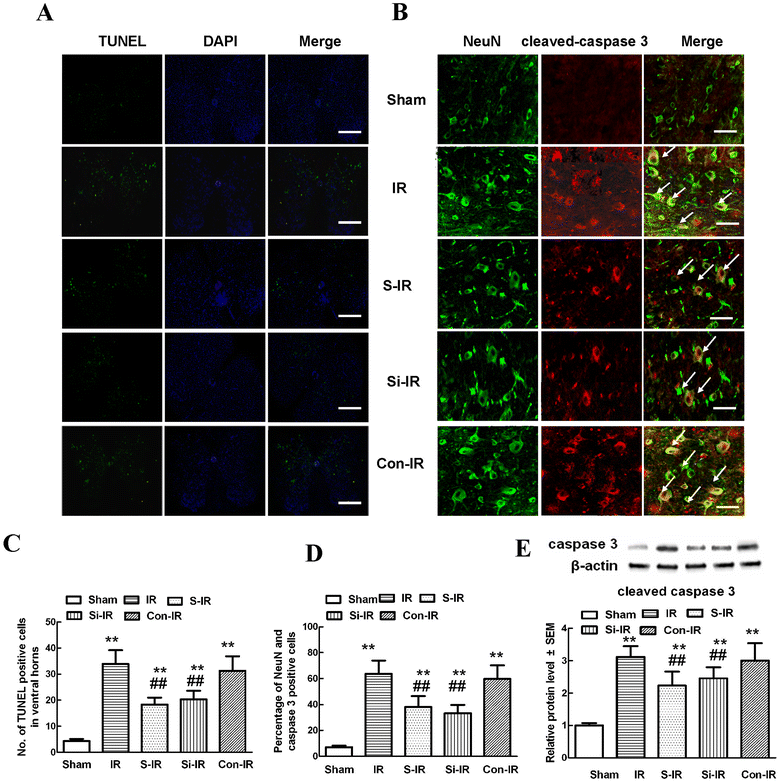
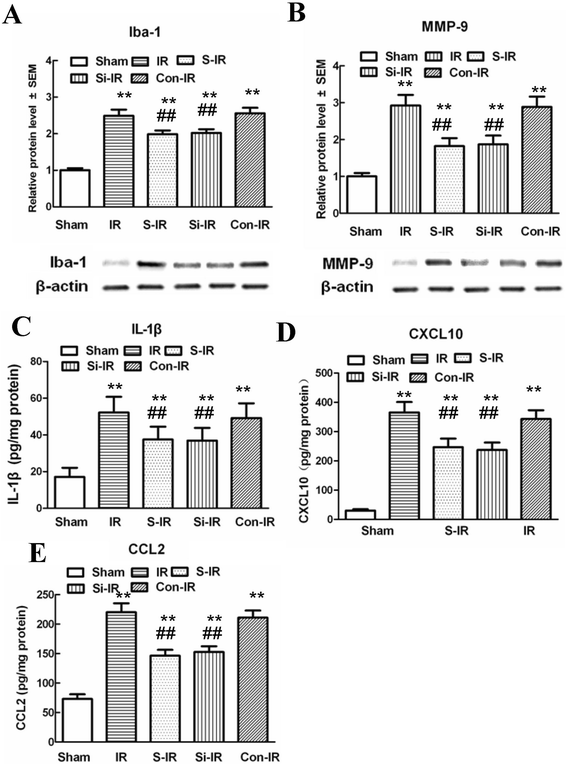
Results: The results showed significant upregulation of MMP-9 in rats with IR-induced inflammation of the BSCB compared to that of the sham group, manifested as dysfunctional BSCB with increased Evans blue extravasation and reduced expression of occludin protein. Increased MMP-9 expression was also observed to facilitate invasion and migration of activated microglia, imaging as high Iba-1 expression, clustered to neurons in the injured spinal cord, as shown by double immunofluorescence, and increased proinflammatory chemokine production (CXCL10, CCL2). Further, sevoflurane preconditioning markedly improved motor function by ameliorating neuronal apoptosis, as shown by reduced TUNEL-positive cell counts and expression of cleaved caspase-3. These protective effects were probably responsible for downregulation of MMP-9 and maintenance of normal expression of occludin protein indicating BSCB integrity from inflammatory damage, which was confirmed by decreased protein levels of Iba-1 and MMP-9, as well as reduced production of proinflammatory chemokines (CXCL10, CCL2) and proinflammatory cytokines (IL-1β). Intrathecal injection of specific siRNAs targeting MMP-9 had similar protective effects to those of sevoflurane preconditioning.
Conclusions: Preconditioning with 2.4% sevoflurane attenuated spinal cord IR injury by inhibiting recruitment of microglia and secretion of MMP-9; thus inhibiting downstream effects on inflammatory damage to BSCB integrity and neuronal apoptosis.
Figures



References
-
- Aubé B, Lévesque SA, Paré A, Chamma E, Kébir H, Gorina R, Lécuyer MA, Alvarez JI, De Koninck Y, Engelhardt B, Prat A, Côté D, Lacroix S: Neutrophils Mediate Blood-Spinal Cord Barrier Disruption in Demyelinating Neuroinflammatory Diseases.J Immunol 2014, [Epub ahead of print]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

