Brief reports: Lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes
- PMID: 25186209
- PMCID: PMC4270893
- DOI: 10.1002/stem.1835
Brief reports: Lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes
Abstract
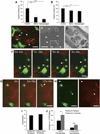
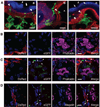
Despite controversies on the potential of hematopoietic stem cells (HSCs) to promote tissue repair, we previously showed that HSC transplantation could correct cystinosis, a multisystemic lysosomal storage disease, caused by a defective lysosomal membrane cystine transporter, cystinosin (CTNS gene). Addressing the cellular mechanisms, we here report vesicular cross-correction after HSC differentiation into macrophages. Upon coculture with cystinotic fibroblasts, macrophages produced tunneling nanotubes (TNTs) allowing transfer of cystinosin-bearing lysosomes into Ctns-deficient cells, which exploited the same route to retrogradely transfer cystine-loaded lysosomes to macrophages, providing a bidirectional correction mechanism. TNT formation was enhanced by contact with diseased cells. In vivo, HSCs grafted to cystinotic kidneys also generated nanotubular extensions resembling invadopodia that crossed the dense basement membranes and delivered cystinosin into diseased proximal tubular cells. This is the first report of correction of a genetic lysosomal defect by bidirectional vesicular exchange via TNTs and suggests broader potential for HSC transplantation for other disorders due to defective vesicular proteins.
Keywords: Cystinosis; Hematopoietic stem cells; Lysosomal cross-correction; Macrophages; Tubular basement membrane; Tunneling nanotubes.
© 2014 AlphaMed Press.
Conflict of interest statement
No support for this work was derived from any commercial source and the authors have no direct financial interest in any aspect of the manuscript.
Figures

Similar articles
-
Macrophage polarization impacts tunneling nanotube formation and intercellular organelle trafficking.Sci Rep. 2019 Oct 10;9(1):14529. doi: 10.1038/s41598-019-50971-x. Sci Rep. 2019. PMID: 31601865 Free PMC article.
-
Hematopoietic Stem Cells Transplantation Can Normalize Thyroid Function in a Cystinosis Mouse Model.Endocrinology. 2016 Apr;157(4):1363-71. doi: 10.1210/en.2015-1762. Epub 2016 Jan 26. Endocrinology. 2016. PMID: 26812160 Free PMC article.
-
Deficiency of the sedoheptulose kinase (Shpk) does not alter the ability of hematopoietic stem cells to rescue cystinosis in the mouse model.Mol Genet Metab. 2021 Dec;134(4):309-316. doi: 10.1016/j.ymgme.2021.11.006. Epub 2021 Nov 17. Mol Genet Metab. 2021. PMID: 34823997 Free PMC article.
-
Hematopoietic Stem Cell Gene Therapy for Cystinosis: From Bench-to-Bedside.Cells. 2021 Nov 23;10(12):3273. doi: 10.3390/cells10123273. Cells. 2021. PMID: 34943781 Free PMC article. Review.
-
Potential use of stem cells as a therapy for cystinosis.Pediatr Nephrol. 2019 Jun;34(6):965-973. doi: 10.1007/s00467-018-3974-7. Epub 2018 May 22. Pediatr Nephrol. 2019. PMID: 29789935 Free PMC article. Review.
Cited by
-
Macrophage polarization impacts tunneling nanotube formation and intercellular organelle trafficking.Sci Rep. 2019 Oct 10;9(1):14529. doi: 10.1038/s41598-019-50971-x. Sci Rep. 2019. PMID: 31601865 Free PMC article.
-
Tuberculosis Exacerbates HIV-1 Infection through IL-10/STAT3-Dependent Tunneling Nanotube Formation in Macrophages.Cell Rep. 2019 Mar 26;26(13):3586-3599.e7. doi: 10.1016/j.celrep.2019.02.091. Cell Rep. 2019. PMID: 30917314 Free PMC article.
-
Tunneling Nanotubes and the Eye: Intercellular Communication and Implications for Ocular Health and Disease.Biomed Res Int. 2020 Apr 8;2020:7246785. doi: 10.1155/2020/7246785. eCollection 2020. Biomed Res Int. 2020. PMID: 32352005 Free PMC article. Review.
-
The spread of prion-like proteins by lysosomes and tunneling nanotubes: Implications for neurodegenerative diseases.J Cell Biol. 2017 Sep 4;216(9):2633-2644. doi: 10.1083/jcb.201701047. Epub 2017 Jul 19. J Cell Biol. 2017. PMID: 28724527 Free PMC article. Review.
-
Evaluation of the efficacy of cystinosin supplementation through CTNS mRNA delivery in experimental models for cystinosis.Sci Rep. 2023 Nov 28;13(1):20961. doi: 10.1038/s41598-023-47085-w. Sci Rep. 2023. PMID: 38016974 Free PMC article.
References
-
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. - PubMed
-
- Ratajczak MZ, Kucia M, Jadczyk T, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. - PubMed
-
- Nevo N, Chol M, Bailleux A, et al. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol Dial Transplant. 2009 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

