Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface
- PMID: 25186731
- PMCID: PMC4224615
- DOI: 10.1038/nature13601
Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface
Abstract
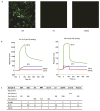
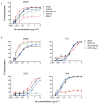
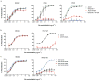
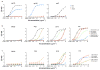
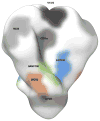
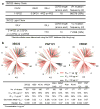
The isolation of human monoclonal antibodies is providing important insights into the specificities that underlie broad neutralization of HIV-1 (reviewed in ref. 1). Here we report a broad and extremely potent HIV-specific monoclonal antibody, termed 35O22, which binds a novel HIV-1 envelope glycoprotein (Env) epitope. 35O22 neutralized 62% of 181 pseudoviruses with a half-maximum inhibitory concentration (IC50) <50 μg ml(-1). The median IC50 of neutralized viruses was 0.033 μg ml(-1), among the most potent thus far described. 35O22 did not bind monomeric forms of Env tested, but did bind the trimeric BG505 SOSIP.664. Mutagenesis and a reconstruction by negative-stain electron microscopy of the Fab in complex with trimer revealed that it bound to a conserved epitope, which stretched across gp120 and gp41. The specificity of 35O22 represents a novel site of vulnerability on HIV Env, which serum analysis indicates to be commonly elicited by natural infection. Binding to this new site of vulnerability may thus be an important complement to current monoclonal-antibody-based approaches to immunotherapies, prophylaxis and vaccine design.
Conflict of interest statement
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
Figures





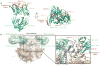

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- UM1 AI100645/AI/NIAID NIH HHS/United States
- AI93278/AI/NIAID NIH HHS/United States
- ZIA AI000855/ImNIH/Intramural NIH HHS/United States
- AI84714/AI/NIAID NIH HHS/United States
- P01 AI082362/AI/NIAID NIH HHS/United States
- 280829/ERC_/European Research Council/International
- UM1 AI100663/AI/NIAID NIH HHS/United States
- ZIA AI001090/ImNIH/Intramural NIH HHS/United States
- R33 AI084714/AI/NIAID NIH HHS/United States
- R01 AI100790/AI/NIAID NIH HHS/United States
- R01 AI093278/AI/NIAID NIH HHS/United States
- R21 AI084714/AI/NIAID NIH HHS/United States

