An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex
- PMID: 25186741
- PMCID: PMC4152602
- DOI: 10.1523/JNEUROSCI.1860-14.2014
An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex
Erratum in
- J Neurosci. 2015 Jan 14;35(2):846-6
Abstract
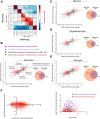
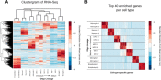
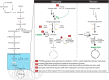
The major cell classes of the brain differ in their developmental processes, metabolism, signaling, and function. To better understand the functions and interactions of the cell types that comprise these classes, we acutely purified representative populations of neurons, astrocytes, oligodendrocyte precursor cells, newly formed oligodendrocytes, myelinating oligodendrocytes, microglia, endothelial cells, and pericytes from mouse cerebral cortex. We generated a transcriptome database for these eight cell types by RNA sequencing and used a sensitive algorithm to detect alternative splicing events in each cell type. Bioinformatic analyses identified thousands of new cell type-enriched genes and splicing isoforms that will provide novel markers for cell identification, tools for genetic manipulation, and insights into the biology of the brain. For example, our data provide clues as to how neurons and astrocytes differ in their ability to dynamically regulate glycolytic flux and lactate generation attributable to unique splicing of PKM2, the gene encoding the glycolytic enzyme pyruvate kinase. This dataset will provide a powerful new resource for understanding the development and function of the brain. To ensure the widespread distribution of these datasets, we have created a user-friendly website (http://web.stanford.edu/group/barres_lab/brain_rnaseq.html) that provides a platform for analyzing and comparing transciption and alternative splicing profiles for various cell classes in the brain.
Keywords: alternative splicing; astrocytes; microglia; oligodendrocytes; transcriptome; vascular cells.
Copyright © 2014 the authors 0270-6474/14/3411929-19$15.00/0.
Figures




References
-
- Bainbridge MN, Warren RL, Hirst M, Romanuik T, Zeng T, Go A, Delaney A, Griffith M, Hickenbotham M, Magrini V, Mardis ER, Sadar MD, Siddiqui AS, Marra MA, Jones SJ. Analysis of the prostate cancer cell line LNCaP transcriptome using a sequencing-by-synthesis approach. BMC Genomics. 2006;7:246. doi: 10.1186/1471-2164-7-246. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
