Thalamic NMDA receptor function is necessary for patterning of the thalamocortical somatosensory map and for sensorimotor behaviors
- PMID: 25186746
- PMCID: PMC4152605
- DOI: 10.1523/JNEUROSCI.1663-14.2014
Thalamic NMDA receptor function is necessary for patterning of the thalamocortical somatosensory map and for sensorimotor behaviors
Abstract
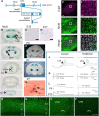
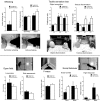
NMDARs play a major role in patterning of topographic sensory maps in the brain. Genetic knock-out of the essential subunit of NMDARs in excitatory cortical neurons prevents whisker-specific neural pattern formation in the barrel cortex. To determine the role of NMDARs en route to the cortex, we generated sensory thalamus-specific NR1 (Grin1)-null mice (ThNR1KO). A multipronged approach, using histology, electrophysiology, optical imaging, and behavioral testing revealed that, in these mice, whisker patterns develop in the trigeminal brainstem but do not develop in the somatosensory thalamus. Subsequently, there is no barrel formation in the neocortex yet a partial afferent patterning develops. Whisker stimulation evokes weak cortical activity and presynaptic neurotransmitter release probability is also affected. We found several behavioral deficits in tasks, ranging from sensorimotor to social and cognitive. Collectively, these results show that thalamic NMDARs play a critical role in the patterning of the somatosensory thalamic and cortical maps and their impairment may lead to pronounced behavioral defects.
Keywords: barrel cortex imaging; barreloids; barrels; glutamate; pattern formation.
Copyright © 2014 the authors 0270-6474/14/3412001-14$15.00/0.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
