TDP-43 toxicity proceeds via calcium dysregulation and necrosis in aging Caenorhabditis elegans motor neurons
- PMID: 25186754
- PMCID: PMC4262699
- DOI: 10.1523/JNEUROSCI.2495-13.2014
TDP-43 toxicity proceeds via calcium dysregulation and necrosis in aging Caenorhabditis elegans motor neurons
Abstract
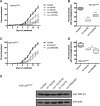
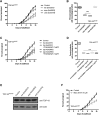
Amyotrophic lateral sclerosis (ALS) is a heterogeneous disease with either sporadic or genetic origins characterized by the progressive degeneration of motor neurons. At the cellular level, ALS neurons show protein misfolding and aggregation phenotypes. Transactive response DNA-binding protein 43 (TDP-43) has recently been shown to be associated with ALS, but the early pathophysiological deficits causing impairment in motor function are unknown. Here we used Caenorhabditis elegans expressing mutant TDP-43(A315T) in motor neurons and explored the potential influences of calcium (Ca(2+)). Using chemical and genetic approaches to manipulate the release of endoplasmic reticulum (ER) Ca(2+)stores, we observed that the reduction of intracellular Ca(2+) ([Ca(2+)]i) rescued age-dependent paralysis and prevented the neurodegeneration of GABAergic motor neurons. Our data implicate elevated [Ca(2+)]i as a driver of TDP-43-mediated neuronal toxicity. Furthermore, we discovered that neuronal degeneration is independent of the executioner caspase CED-3, but instead requires the activity of the Ca(2+)-regulated calpain protease TRA-3, and the aspartyl protease ASP-4. Finally, chemically blocking protease activity protected against mutant TDP-43(A315T)-associated neuronal toxicity. This work both underscores the potential of the C. elegans system to identify key targets for therapeutic intervention and suggests that a focused effort to regulate ER Ca(2+) release and necrosis-like degeneration consequent to neuronal injury may be of clinical importance.
Keywords: ALS; C. elegans; ER; TDP-43; calcium; necrosis.
Copyright © 2014 the authors 0270-6474/14/3412093-11$15.00/0.
Figures






References
-
- Bernard-Marissal N, Moumen A, Sunyach C, Pellegrino C, Dudley K, Henderson CE, Raoul C, Pettmann B. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J Neurosci. 2012;32:4901–4912. doi: 10.1523/JNEUROSCI.5431-11.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
