A randomised controlled trial to assess the effectiveness of a nurse-led palliative care intervention for HIV positive patients on antiretroviral therapy: recruitment, refusal, randomisation and missing data
- PMID: 25187211
- PMCID: PMC4161861
- DOI: 10.1186/1756-0500-7-600
A randomised controlled trial to assess the effectiveness of a nurse-led palliative care intervention for HIV positive patients on antiretroviral therapy: recruitment, refusal, randomisation and missing data
Abstract
Background: Despite the life threatening nature of an HIV diagnosis and the multidimensional problems experienced by this patient population during antiretroviral therapy, the effectiveness of a palliative care approach for HIV positive patients on ART is as yet unknown.
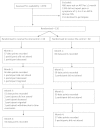
Findings: A randomised controlled trial (RCT) was conducted in a sample of 120 HIV positive patients on ART in an urban clinic in Mombasa, Kenya. The intervention was a minimum of seven sessions of multidimensional, person-centred care, given by HIV nurses trained in the palliative care approach over a period of 5 months. Rates of recruitment and refusal, the effectiveness of the randomisation procedure, trial follow-up and attrition and extent of missing data are reported.120 patients (60 randomised to control arm, 60 randomised to intervention arm) were recruited over 5.5 months, with a refusal rate of 55.7%. During the study period, three participants died from cancer, three withdrew (two moved away and one withdrew due to time constraints). All of these patients were in the intervention arm: details are reported. There were five additional missing monthly interviews in both the control and intervention study arm, bringing the total of missing data to 26 data points (4.3%).
Discussion: The quality and implications of these data are discussed extensively and openly, including the effect of full and ethical consent procedures, respondent burden, HIV stigma, accurate randomisation, patient safety and the impact of the intervention. Data on recruitment randomisation, attrition and missing data in clinical trials should be routinely reported, in conjunction with the now established practice of publishing study protocols to enhance research integrity, transparency and quality. Transparency is especially important in cross cultural settings, in which the sources of funding and trial design are often not based in the country of data collection. Findings reported can be used to inform future RCTs in this area.
Trial registration: Clinicaltrials.gov NCT01608802.
Figures
References
-
- Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2010. Geneva, Switzerland: WHO, UNICEF; 2010. Available from http://whqlibdoc.who.int/publications/2010/9789241500395_eng.pdf
-
- PEPFAR . HIV/AIDS Palliative Care Guidance#1 for the United States Government in–Country Staff And Implementing Partners. 2006.
-
- UNICEF . Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. Geneva, Switzerland: World Health Organization; 2011.
-
- Consolidated guidelines on general HIV care and the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: WHO; 2013. Available from http://www.who.int/hiv/pub/guidelines/arv2013/en/ - PubMed
-
- Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. Geneva: WHO; 2010. Available from http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical