Biological activities of fusarochromanone: a potent anti-cancer agent
- PMID: 25187308
- PMCID: PMC4168212
- DOI: 10.1186/1756-0500-7-601
Biological activities of fusarochromanone: a potent anti-cancer agent
Abstract
Background: Fusarochromanone (FC101) is a small molecule fungal metabolite with a host of interesting biological functions, including very potent anti-angiogenic and direct anti-cancer activity.
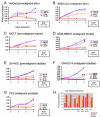
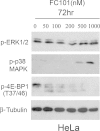
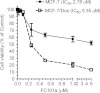
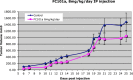
Results: Herein, we report that FC101 exhibits very potent in-vitro growth inhibitory effects (IC50 ranging from 10nM-2.5 μM) against HaCat (pre-malignant skin), P9-WT (malignant skin), MCF-7 (low malignant breast), MDA-231 (malignant breast), SV-HUC (premalignant bladder), UM-UC14 (malignant bladder), and PC3 (malignant prostate) in a time-course and dose-dependent manner, with the UM-UC14 cells being the most sensitive. FC101 induces apoptosis and an increase in proportion of cells in the sub-G1 phase in both HaCat and P9-WT cell lines as evidenced by cell cycle profile analysis. In a mouse xenograft SCC tumor model, FC101 was well tolerated, non-toxic, and achieved a 30% reduction in tumor size at a dose of 8 mg/kg/day. FC101 is also a potent anti-angiogenenic agent. At nanomolar doses, FC101 inhibits the vascular endothelial growth factor-A (VEGF-A)-mediated proliferation of endothelial cells.
Conclusions: Our data presented here indicates that FC101 is an excellent lead candidate for a small molecule anti-cancer agent that simultaneously affects angiogenesis signaling, cancer signal transduction, and apoptosis. Further understanding of the underlying FC101's molecular mechanism may lead to the design of novel targeted and selective therapeutics, both of which are pursued targets in cancer drug discovery.
Figures





References
-
- Miloslav R. Handbook of Foodborne Diseases of Biological Origin (CRC Series in Nutrition and Food) Boca Raton, Florida: CRC Press; 1983. p. 473.
-
- Pathre SV, Gleason WB, Lee Y-W, Mirocha CJ. The Structure of fusarochromanone: New mycotoxin from Fusarium roseum. Gramineurum. Can J Chem. 1986;64:1308. doi: 10.1139/v86-217. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

