Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens
- PMID: 25187361
- PMCID: PMC4274804
- DOI: 10.2337/db13-1639
Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens
Abstract
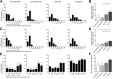
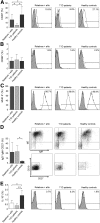
Impaired regulatory B cell (Breg) responses are associated with several autoimmune diseases in humans; however, the role of Bregs in type 1 diabetes (T1D) remains unclear. We hypothesized that naturally occurring, interleukin-10 (IL-10)-producing Bregs maintain tolerance to islet autoantigens, and that hyperglycemic nonobese diabetic (NOD) mice and T1D patients lack these potent negative regulators. IgVH transcriptome analysis revealed that islet-infiltrating B cells in long-term normoglycemic (Lnglc) NOD, which are naturally protected from diabetes, are more antigen-experienced and possess more diverse B-cell receptor repertoires compared to those of hyperglycemic (Hglc) mice. Importantly, increased levels of Breg-promoting CD40(+) B cells and IL-10-producing B cells were found within islets of Lnglc compared to Hglc NOD. Likewise, healthy individuals showed increased frequencies of both CD40(+) and IL-10(+) B cells compared to T1D patients. Rituximab-mediated B-cell depletion followed by adoptive transfer of B cells from Hglc mice induced hyperglycemia in Lnglc human CD20 transgenic NOD mouse models. Importantly, both murine and human IL-10(+) B cells significantly abrogated T-cell-mediated responses to self- or islet-specific peptides ex vivo. Together, our data suggest that antigen-matured Bregs may maintain tolerance to islet autoantigens by selectively suppressing autoreactive T-cell responses, and that Hglc mice and individuals with T1D lack this population of Bregs.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures





References
-
- Bluestone JA, Pardoll D, Sharrow SO, Fowlkes BJ. Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature 1987;326:82–84 - PubMed
-
- Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes 1997;46:941–946 - PubMed
-
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature 1985;314:537–539 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

