Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes
- PMID: 25187362
- PMCID: PMC4274803
- DOI: 10.2337/db14-0215
Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes
Abstract
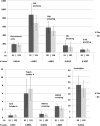
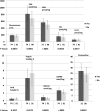
Six skin collagen advanced glycation end products (AGEs) originally measured near to the time of the Diabetes Control and Complications Trial (DCCT) closeout in 1993 may contribute to the "metabolic memory" phenomenon reported in the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study. We have now investigated whether the addition of four originally unavailable AGEs (i.e., glucosepane [GSPNE], hydroimidazolones of methylglyoxal [MG-H1] and glyoxal, and carboxyethyl-lysine) improves associations with incident retinopathy, nephropathy, and neuropathy events during 13-17 years after DCCT. The complete 10-AGE panel is associated with three-step Early Treatment of Diabetic Retinopathy Study scale worsening of retinopathy (P ≤ 0.002), independent of either mean DCCT or EDIC study A1C level. GSPNE and fructose-lysine (furosine [FUR]) correlate with retinopathy progression, independently of A1C level. The complete panel also correlates with microalbuminuria (P = 0.008) and FUR with nephropathy independently of A1C level (P ≤ 0.02). Neuropathy correlates with the complete panel despite adjustment for A1C level (P ≤ 0.005). MG-H1 and FUR are dominant, independent of A1C level (P < 0.0001), whereas A1C loses significance after adjustment for the AGEs. Overall, the added set of four AGEs enhances the association of the original panel with progression risk of retinopathy and neuropathy (P < 0.04) but not nephropathy, while GSPNE and MG-H1 emerge as the principal new risk factors. Skin AGEs are robust long-term markers of microvascular disease progression, emphasizing the importance of early and sustained implementation of intensive therapy.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

References
-
- Genuth SM. The case for blood glucose control. Adv Intern Med 1995;40:573–623 - PubMed
-
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- The DCCT Research Group . The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 1995;44:968–983 - PubMed
-
- Monnier VM, Sell DR, Genuth S. Glycation products as markers and predictors of the progression of diabetic complications. Ann N Y Acad Sci 2005;1043:567–581 - PubMed
-
- Monnier VM, Bautista O, Kenny D, et al. . Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–880 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

