Translocation of glycogen synthase kinase-3β (GSK-3β), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2)
- PMID: 25187518
- PMCID: PMC4200279
- DOI: 10.1074/jbc.M114.563924
Translocation of glycogen synthase kinase-3β (GSK-3β), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2)
Abstract
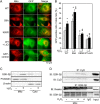
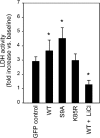
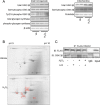
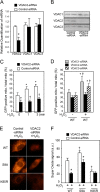
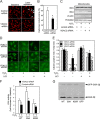
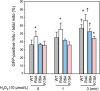
Glycogen synthase kinase-3β (GSK-3β) is a major positive regulator of the mitochondrial permeability transition pore (mPTP), a principle trigger of cell death, under the condition of oxidative stress. However, the mechanism by which cytosolic GSK-3β translocates to mitochondria, promoting mPTP opening, remains unclear. Here we addressed this issue by analyses of the effect of site-directed mutations in GSK-3β on mitochondrial translocation and protein/protein interactions upon oxidative stress. H9c2 cardiomyoblasts were transfected with GFP-tagged GSK-3β (WT), a mutant GSK-3β insensitive to inhibitory phosphorylation (S9A), or kinase-deficient GSK-3β (K85R). Time lapse observation revealed that WT and S9A translocated from the cytosol to the mitochondria more promptly than did K85R after exposure to oxidative stress. H2O2 increased the density of nine spots on two-dimensional gel electrophoresis of anti-GSK-3β-immunoprecipitates by more than 3-fold. MALDI-TOF/MS analysis revealed that one of the spots contained voltage-dependent anion channel 2 (VDAC2). Knockdown of VDAC2, but not VDAC1 or VDAC3, by siRNA attenuated both the mitochondrial translocation of GSK-3β and mPTP opening under stress conditions. The mitochondrial translocation of GSK-3β was attenuated also when Lys-15, but not Arg-4 or Arg-6, in the N-terminal domain of GSK-3β was replaced with alanine. The oxidative stress-induced mitochondrial translocation of GSK-3β was associated with an increase in cell death, which was suppressed by lithium chloride (LiCl), a GSK-3β inhibitor. These results demonstrate that GSK-3β translocates from the cytosol to mitochondria in a kinase activity- and VDAC2-dependent manner in which an N-terminal domain of GSK-3β may function as a mitochondrial targeting sequence.
Keywords: Glycogen Synthase Kinase 3 (GSK-3); Mitochondrial Permeability Transition (MPT); Mitochondrial Transport; Necrosis (Necrotic Death); Protein-Protein Interaction; Voltage-dependent Anion Channel 2.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Miura T., Tanno M. (2012) The mPTP and its regulatory proteins: final common targets of signalling pathways for protection against necrosis. Cardiovasc. Res. 94, 181–189 - PubMed
-
- Weiss J. N., Korge P., Honda H. M., Ping P. (2003) Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 93, 292–301 - PubMed
-
- Halestrap A. P. (2009) What is the mitochondrial permeability transition pore? J Mol. Cell. Cardiol. 46, 821–831 - PubMed
-
- Miura T., Tanno M., Sato T. (2010) Mitochondrial kinase signalling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc. Res. 88, 7–15 - PubMed
-
- Halestrap A. P. (2010) A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 38, 841–860 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

