The relevance and potential roles of microphysiological systems in biology and medicine
- PMID: 25187571
- PMCID: PMC4330974
- DOI: 10.1177/1535370214542068
The relevance and potential roles of microphysiological systems in biology and medicine
Abstract
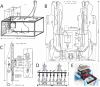
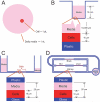
Microphysiological systems (MPS), consisting of interacting organs-on-chips or tissue-engineered, 3D organ constructs that use human cells, present an opportunity to bring new tools to biology, medicine, pharmacology, physiology, and toxicology. This issue of Experimental Biology and Medicine describes the ongoing development of MPS that can serve as in-vitro models for bone and cartilage, brain, gastrointestinal tract, lung, liver, microvasculature, reproductive tract, skeletal muscle, and skin. Related topics addressed here are the interconnection of organs-on-chips to support physiologically based pharmacokinetics and drug discovery and screening, and the microscale technologies that regulate stem cell differentiation. The initial motivation for creating MPS was to increase the speed, efficiency, and safety of pharmaceutical development and testing, paying particular regard to the fact that neither monolayer monocultures of immortal or primary cell lines nor animal studies can adequately recapitulate the dynamics of drug-organ, drug-drug, and drug-organ-organ interactions in humans. Other applications include studies of the effect of environmental toxins on humans, identification, characterization, and neutralization of chemical and biological weapons, controlled studies of the microbiome and infectious disease that cannot be conducted in humans, controlled differentiation of induced pluripotent stem cells into specific adult cellular phenotypes, and studies of the dynamics of metabolism and signaling within and between human organs. The technical challenges are being addressed by many investigators, and in the process, it seems highly likely that significant progress will be made toward providing more physiologically realistic alternatives to monolayer monocultures or whole animal studies. The effectiveness of this effort will be determined in part by how easy the constructs are to use, how well they function, how accurately they recapitulate and report human pharmacology and toxicology, whether they can be generated in large numbers to enable parallel studies, and if their use can be standardized consistent with the practices of regulatory science.
Keywords: Organs on chips; drug discovery and development; drug safety and toxicity; drug–organ interactions; environmental toxicology; induced pluripotent stem cells; microphysiological systems; quantitative systems pharmacology; systems biology; tissue-engineered organ constructs.
© The Author(s) 2014 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav.
Figures


References
-
- Martin HN. The direct influence of gradual variations of temperature upon the rate of beat of the dog’s heart. Philos Trans R Soc London. 1883 Jan 1;174:663–88.
-
- Langendorff O. Untersuchungen am Überlebenden Säugethierherzen. Pflug Arch Eur J Phy. 1895;61(6):291–332.
-
- Avis FR. Investigations of liver and kidney. Design of a dual apparatus for research study. Science Teacher. 1961;28(1):14–8.
-
- Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967 Apr 1;212(4):804–14. - PubMed
-
- Neely JR, Liebermeister H, Morgan HE. Effect of pressure development on membrane transport of glucose in isolated rat heart. Am J Physiol. 1967;212(4):815–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

