The O-glycomap of lubricin, a novel mucin responsible for joint lubrication, identified by site-specific glycopeptide analysis
- PMID: 25187573
- PMCID: PMC4256492
- DOI: 10.1074/mcp.M114.040865
The O-glycomap of lubricin, a novel mucin responsible for joint lubrication, identified by site-specific glycopeptide analysis
Abstract
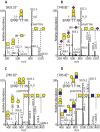
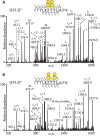
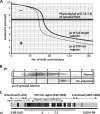
The lubricative, heavily glycosylated mucin-like synovial glycoprotein lubricin has previously been observed to contain glycosylation changes related to rheumatoid and osteoarthritis. Thus, a site-specific investigation of the glycosylation of lubricin was undertaken, in order to further understand the pathological mechanisms involved in these diseases. Lubricin contains an serine/threonine/proline (STP)-rich domain composed of imperfect tandem repeats (EPAPTTPK), the target for O-glycosylation. In this study, using a liquid chromatography-tandem mass spectrometry approach, employing both collision-induced and electron-transfer dissociation fragmentation methods, we identified 185 O-glycopeptides within the STP-rich domain of human synovial lubricin. This showed that adjacent threonine residues within the central STP-rich region could be simultaneously and/or individually glycosylated. In addition to core 1 structures responsible for biolubrication, core 2 O-glycopeptides were also identified, indicating that lubricin glycosylation may have other roles. Investigation of the expression of polypeptide N-acetylgalactosaminyltransferase genes was carried out using cultured primary fibroblast-like synoviocytes, a cell type that expresses lubricin in vivo. This analysis showed high mRNA expression levels of the less understood polypeptide N-acetylgalactosaminyltransferase 15 and 5 in addition to the ubiquitously expressed polypeptide N-acetylgalactosaminyltransferase 1 and 2 genes. This suggests that there is a unique combination of transferase genes important for the O-glycosylation of lubricin. The site-specific glycopeptide analysis covered 82% of the protein sequence and showed that lubricin glycosylation displays both micro- and macroheterogeneity. The density of glycosylation was shown to be high: 168 sites of O-glycosylation, predominately sialylated, were identified. These glycosylation sites were focused in the central STP-rich region, giving the domain a negative charge. The more positively charged lysine and arginine residues in the N and C termini suggest that synovial lubricin exists as an amphoteric molecule. The identification of these unique properties of lubricin may provide insight into the important low-friction lubricating functions of lubricin during natural joint movement.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Jay G. D., Tantravahi U., Britt D. E., Barrach H. J., Cha C. J. (2001) Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J. Orthop. Res 19, 677–687 - PubMed
-
- Jay G. D., Fleming B. C., Watkins B. A., McHugh K. A., Anderson S. C., Zhang L. X., Teeple E., Waller K. A., Elsaid K. A. (2010) Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 62, 2382–2391 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

