Neuroimaging diagnosis and the collateral circulation in moyamoya disease
- PMID: 25187770
- PMCID: PMC4031769
- DOI: 10.1159/000346765
Neuroimaging diagnosis and the collateral circulation in moyamoya disease
Abstract
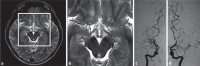
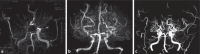
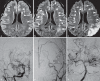
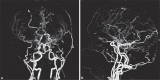
Moyamoya disease (MMD) is an uncommon cerebrovascular disease that is characterized by progressive stenosis of the terminal portion of the internal carotid artery and its main branches, which is accompanied by the development of dilated, fragile collateral vessels at the base of the brain. This review will present different neuroimaging modalities for the diagnosis of MMD. Importantly, we will discuss the role of hyperintense vessels on fluid-attenuated inversion recovery images and their contribution to the evaluation of collateral patterns in MMD patients. Additionally, this review will summarize these common collateral patterns of MMD assessed by conventional cerebral angiography, and the associations of these angiographic collateral patterns with cerebrovascular lesions, including ischemia and hemorrhage will also be reviewed.
Keywords: Angiography; Collateral blood flow; Fluid-attenuated inversion recovery; Hyperintense vessels; Moyamoya disease.
Figures


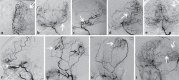
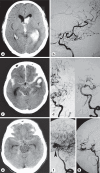
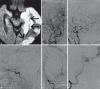
References
-
- Suzuki J, Takaku A. Cerebrovascular ‘moyamoya’ disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–299. - PubMed
-
- Liu W, Zhu S, Wang X, Yue X, Zhou Zhi, Wang H, Xu G, Zhou C, Liu X. Evaluation of angiographic changes of the anterior choroidal and posterior communicating arteries for predicting cerebrovascular lesions in adult moyamoya disease. J Clin Neurosci. 2011;18:374–378. - PubMed
-
- Harada A, Fujii Y, Yoneoka Y, Takeuchi S, Tanaka R, Nakada T. High-field magnetic resonance imaging in patients with moyamoya disease. J Neurosurg. 2001;94:233–237. - PubMed
-
- Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997;99(suppl 2):S238–S240. - PubMed
-
- Houkin K, Aoki T, Takahashi A, Abe H. Diagnosis of moyamoya disease with magnetic resonance angiography. Stroke. 1994;25:2159–2164. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

