A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis
- PMID: 25187999
- PMCID: PMC4304638
- DOI: 10.1097/ALN.0000000000000419
A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis
Erratum in
- Anesthesiology. 2015 Apr;122(4):958. Lei, Gao [corrected to Gao, Lei]
Abstract
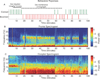
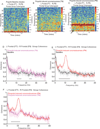
Background: Electroencephalogram patterns observed during sedation with dexmedetomidine appear similar to those observed during general anesthesia with propofol. This is evident with the occurrence of slow (0.1 to 1 Hz), delta (1 to 4 Hz), propofol-induced alpha (8 to 12 Hz), and dexmedetomidine-induced spindle (12 to 16 Hz) oscillations. However, these drugs have different molecular mechanisms and behavioral properties and are likely accompanied by distinguishing neural circuit dynamics.
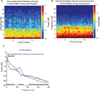
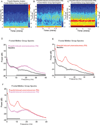
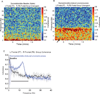
Methods: The authors measured 64-channel electroencephalogram under dexmedetomidine (n = 9) and propofol (n = 8) in healthy volunteers, 18 to 36 yr of age. The authors administered dexmedetomidine with a 1-µg/kg loading bolus over 10 min, followed by a 0.7 µg kg h infusion. For propofol, the authors used a computer-controlled infusion to target the effect-site concentration gradually from 0 to 5 μg/ml. Volunteers listened to auditory stimuli and responded by button press to determine unconsciousness. The authors analyzed the electroencephalogram using multitaper spectral and coherence analysis.
Results: Dexmedetomidine was characterized by spindles with maximum power and coherence at approximately 13 Hz (mean ± SD; power, -10.8 ± 3.6 dB; coherence, 0.8 ± 0.08), whereas propofol was characterized with frontal alpha oscillations with peak frequency at approximately 11 Hz (power, 1.1 ± 4.5 dB; coherence, 0.9 ± 0.05). Notably, slow oscillation power during a general anesthetic state under propofol (power, 13.2 ± 2.4 dB) was much larger than during sedative states under both propofol (power, -2.5 ± 3.5 dB) and dexmedetomidine (power, -0.4 ± 3.1 dB).
Conclusion: The results indicate that dexmedetomidine and propofol place patients into different brain states and suggest that propofol enables a deeper state of unconsciousness by inducing large-amplitude slow oscillations that produce prolonged states of neuronal silence.
Conflict of interest statement
All other authors have no conflict of interest.
Figures


Comment in
-
Propofol-induced electroencephalogram dynamics: a missing piece.Anesthesiology. 2015 Sep;123(3):723-5. doi: 10.1097/ALN.0000000000000793. Anesthesiology. 2015. PMID: 26284862 No abstract available.
-
In reply.Anesthesiology. 2015 Sep;123(3):725-8. doi: 10.1097/ALN.0000000000000794. Anesthesiology. 2015. PMID: 26284863 Free PMC article. No abstract available.
References
-
- Gibbs FA, Gibbs EL, Lennox WG. Effect on the electroencephalogram of certain drugs which influence nervous activity. Arch Intern Med. 1937;60:154–169.
-
- Kiersey DK, Bickford RG, Faulconer A., Jr Electro-encephalographic patterns produced by thiopental sodium during surgical operations; description and classification. BJA. 1951;23:141–152. - PubMed
-
- Faulconer A. Correlation of concentrations of ether in arterial blood with electroencephalographic patterns occurring during ether-oxygen and during nitrous oxide, oxygen and ether anesthesia of human surgical patients. Anesthesiology. 1952;13:361–369. - PubMed
-
- Palanca BJ, Mashour GA, Avidan MS. Processed electroencephalogram in depth of anesthesia monitoring. Curr Opin Anaesthesiol. 2009;22:553–559. - PubMed
-
- Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

