48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1-infected patients: a systematic review and network meta-analysis
- PMID: 25188312
- PMCID: PMC4154896
- DOI: 10.1371/journal.pone.0105653
48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1-infected patients: a systematic review and network meta-analysis
Abstract
Background: A network meta-analysis can provide estimates of relative efficacy for treatments not directly studied in head-to-head randomized controlled trials. We estimated the relative efficacy and safety of dolutegravir (DTG) versus third agents currently recommended by guidelines, including ritonavir-boosted atazanavir (ATV/r), ritonavir-boosted darunavir (DRV/r), efavirenz (EFV), cobicistat-boosted elvitegravir (EVG/c), ritonavir-boosted lopinavir (LPV/r), raltegravir (RAL), and rilpivirine (RPV), in treatment-naive HIV-1-infected patients.
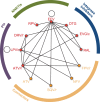
Methods: A systematic review of published literature was conducted to identify phase 3/4 randomized controlled clinical trials (up to August 2013) including at least one third agent of interest in combination with a backbone nucleoside reverse transcriptase inhibitor (NRTI) regimen. Bayesian fixed-effect network meta-analysis models adjusting for the type of nucleoside reverse transcriptase inhibitor backbone (tenofovir disoproxil fumarate/emtricitabine [TDF/FTC] or abacavir/lamivudine [ABC/3TC]) were used to evaluate week 48 efficacy (HIV-RNA suppression to <50 copies/mL and change in CD4+ cells/µL) and safety (lipid changes, adverse events, and discontinuations due to adverse events) of DTG relative to all other treatments. Sensitivity analyses assessing the impact of NRTI treatment adjustment and random-effects models were performed.
Results: Thirty-one studies including 17,000 patients were combined in the analysis. Adjusting for the effect of NRTI backbone, treatment with DTG resulted in significantly higher odds of virologic suppression (HIV RNA<50 copies/mL) and increase in CD4+ cells/µL versus ATV/r, DRV/r, EFV, LPV/r, and RPV. Dolutegravir had better or equivalent changes in total cholesterol, LDL, triglycerides, and lower odds of adverse events and discontinuation due to adverse events compared to all treatments. Random-effects and unadjusted models resulted in similar conclusions.
Conclusion: Three clinical trials of DTG have demonstrated comparable or superior efficacy and safety to DRV, RAL, and EFV in HIV-1-infected treatment-naive patients. This network meta-analysis suggests DTG is also favorable or comparable to other commonly used third agents (ATV/r, LPV/r, RPV, and EVG/c).
Conflict of interest statement
Figures



References
-
- European AIDS Clinical Society (2013) Guidelines, Version 7.0. Available: http://www.eacsociety.org/Portals/0/Guidelines_Online_131014.pdf. Accessed 2014 Jun 24.
-
- Department of Health and Human Services (2014) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 2014 Jun 24.
-
- Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, et al. (2013) Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 27: 973–979. - PubMed
-
- Fong R, Cheng AC, Vujovic O, Hoy JF (2013) Factors associated with virological failure in a cohort of combination antiretroviral therapy-treated patients managed at a tertiary referral centre. Sex Health 10: 442–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

