Targeting Btk/Etk of prostate cancer cells by a novel dual inhibitor
- PMID: 25188519
- PMCID: PMC4540187
- DOI: 10.1038/cddis.2014.343
Targeting Btk/Etk of prostate cancer cells by a novel dual inhibitor
Abstract
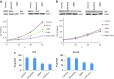
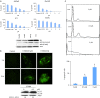
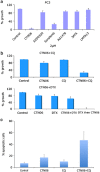
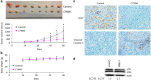
Btk and Etk/BMX are Tec-family non-receptor tyrosine kinases. Btk has previously been reported to be expressed primarily in B cells and has an important role in immune responses and B-cell malignancies. Etk has been shown previously to provide a strong survival and metastasis signal in human prostate cancer cells, and to confer androgen independence and drug resistance. While the role of Etk in prostate carcinogenesis is well established, the functions of Btk in prostate cancer have never been investigated, likely due to the perception that Btk is a hematopoietic, but not epithelial, kinase. Herein, we found that Btk is overexpressed in prostate cancer tissues and prostate cancer cells. The level of Btk in prostate cancer tissues correlates with cancer grades. Knockdown of Btk expression selectively inhibits the growth of prostate cancer cells, but not that of the normal prostate epithelial cells, which express very little Btk. Dual inhibition of Btk and Etk has an additive inhibitory effect on prostate cancer cell growth. To explore Btk and Etk as targets for prostate cancer, we developed a small molecule dual inhibitor of Btk and Etk, CTN06. Treatment of PC3 and other prostate cancer cells, but not immortalized prostate epithelial cells with CTN06 resulted in effective cell killing, accompanied by the attenuation of Btk/Etk signals. The killing effect of CTN06 is more potent than that of commonly used inhibitors against Src, Raf/VEGFR and EGFR. CTN06 induces apoptosis as well as autophagy in human prostate cancer cells, and is a chemo-sensitizer for docetaxel (DTX), a standard of care for metastatic prostate cancer patients. CTN06 also impeded the migration of human prostate cancer cells based on a 'wound healing' assay. The anti-cancer effect of CTN06 was further validated in vivo in a PC3 xenograft mouse model.
Figures



Similar articles
-
CTA095, a novel Etk and Src dual inhibitor, induces apoptosis in prostate cancer cells and overcomes resistance to Src inhibitors.PLoS One. 2013 Aug 15;8(8):e70910. doi: 10.1371/journal.pone.0070910. eCollection 2013. PLoS One. 2013. PMID: 23967135 Free PMC article.
-
Tyrosine kinase Etk/BMX is up-regulated in human prostate cancer and its overexpression induces prostate intraepithelial neoplasia in mouse.Cancer Res. 2006 Aug 15;66(16):8058-64. doi: 10.1158/0008-5472.CAN-06-1364. Cancer Res. 2006. PMID: 16912182
-
Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3'-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells.Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3644-9. doi: 10.1073/pnas.95.7.3644. Proc Natl Acad Sci U S A. 1998. PMID: 9520419 Free PMC article.
-
Targeting tyrosine kinases and autophagy in prostate cancer.Horm Cancer. 2011 Feb;2(1):38-46. doi: 10.1007/s12672-010-0053-3. Epub 2010 Dec 2. Horm Cancer. 2011. PMID: 21350583 Free PMC article. Review.
-
Targeting Small Molecule Tyrosine Kinases by Polyphenols: New Move Towards Anti-tumor Drug Discovery.Curr Drug Discov Technol. 2020;17(5):585-615. doi: 10.2174/1570163816666190808120843. Curr Drug Discov Technol. 2020. PMID: 31393251 Review.
Cited by
-
Genome-wide CRISPR/Cas9 knockout screening uncovers a novel inflammatory pathway critical for resistance to arginine-deprivation therapy.Theranostics. 2021 Jan 25;11(8):3624-3641. doi: 10.7150/thno.51795. eCollection 2021. Theranostics. 2021. PMID: 33664852 Free PMC article.
-
The intensification of anticancer activity of LFM-A13 by erythropoietin as a possible option for inhibition of breast cancer.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1697-1711. doi: 10.1080/14756366.2020.1818738. J Enzyme Inhib Med Chem. 2020. PMID: 32912025 Free PMC article.
-
Targeting Solid Tumors With BTK Inhibitors.Front Cell Dev Biol. 2021 Apr 14;9:650414. doi: 10.3389/fcell.2021.650414. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937249 Free PMC article.
-
Erythropoietin Intensifies the Proapoptotic Activity of LFM-A13 in Cells and in a Mouse Model of Colorectal Cancer.Int J Mol Sci. 2018 Apr 23;19(4):1262. doi: 10.3390/ijms19041262. Int J Mol Sci. 2018. PMID: 29690619 Free PMC article.
-
BTK Isoforms p80 and p65 Are Expressed in Head and Neck Squamous Cell Carcinoma (HNSCC) and Involved in Tumor Progression.Cancers (Basel). 2023 Jan 3;15(1):310. doi: 10.3390/cancers15010310. Cancers (Basel). 2023. PMID: 36612306 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. - PubMed
-
- Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, et al. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol. 2012;13:369–378. - PubMed
-
- Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. - PubMed
-
- Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–10495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

