Generation of cerebral organoids from human pluripotent stem cells
- PMID: 25188634
- PMCID: PMC4160653
- DOI: 10.1038/nprot.2014.158
Generation of cerebral organoids from human pluripotent stem cells
Abstract
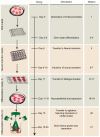
Human brain development exhibits several unique aspects, such as increased complexity and expansion of neuronal output, that have proven difficult to study in model organisms. As a result, in vitro approaches to model human brain development and disease are an intense area of research. Here we describe a recently established protocol for generating 3D brain tissue, so-called cerebral organoids, which closely mimics the endogenous developmental program. This method can easily be implemented in a standard tissue culture room and can give rise to developing cerebral cortex, ventral telencephalon, choroid plexus and retinal identities, among others, within 1-2 months. This straightforward protocol can be applied to developmental studies, as well as to the study of a variety of human brain diseases. Furthermore, as organoids can be maintained for more than 1 year in long-term culture, they also have the potential to model later events such as neuronal maturation and survival.
Figures



Comment in
-
Findings of Research Misconduct.Fed Regist. 2020 Aug 14;85(158):49661-49662. Fed Regist. 2020. PMID: 32831428 Free PMC article. No abstract available.
References
-
- Lanza R, Gearhart J, Hogan B, Melton D, Pedersen R. Essentials of stem cell biology. Elsevier. 2009
-
- Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. - PubMed
-
- Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: self-organising stem cells. Development. 2012;139:4111–4121. - PubMed
-
- Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. - PubMed
-
- Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

