Volatile emanations from in vitro airway cells infected with human rhinovirus
- PMID: 25189196
- PMCID: PMC4182727
- DOI: 10.1088/1752-7155/8/3/037110
Volatile emanations from in vitro airway cells infected with human rhinovirus
Abstract
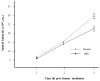
Respiratory viral infections such as human rhinovirus (HRV) can lead to substantial morbidity and mortality, especially in people with underlying lung diseases such as asthma and COPD. One proposed strategy to detect viral infections non-invasively is by volatile organic compound (VOC) assessment via analysis of exhaled breath. The epithelial cells are one of the most important cell lines affected during respiratory infections as they are the first line of pathogen defense. Efforts to discover infection-specific biomarkers can be significantly aided by understanding the VOC emanations of respiratory epithelial cells. Here we test the hypothesis that VOCs obtained from the headspace of respiratory cell culture will differentiate healthy cells from those infected with HRV. Primary human tracheobronchial cells were cultured and placed in a system designed to trap headspace VOCs. HRV-infected cells were compared to uninfected control cells. In addition, cells treated with heat-killed HRV and poly(I:C), a TLR3 agonist, were compared to controls. The headspace was sampled with solid-phase microextraction fibers and VOCs were analyzed by gas chromatography/mass spectrometry. We determined differential expression of compounds such as aliphatic alcohols, branched hydrocarbons, and dimethyl sulfide by the infected cells, VOCs previously associated with oxidative stress and bacterial infection. We saw no major differences between the killed-HRV, poly(I:C), and control cell VOCs. We postulate that these compounds may serve as biomarkers of HRV infection, and that the production of VOCs is not due to TLR3 stimulation but does require active viral replication. Our novel approach may be used for the in vitro study of other important respiratory viruses, and ultimately it may aid in identifying VOC biomarkers of viral infection for point-of-care diagnostics.
Figures



References
-
- AKSENOV AA, GOJOVA A, ZHAO W, MORGAN JT, SANKARAN S, SANDROCK CE, DAVIS CE. Characterization of Volatile Organic Compounds in Human Leukocyte Antigen Heterologous Expression Systems: a Cell’s “Chemical Odor Fingerprint”. Chembiochem. 2012;13:1053–1059. - PubMed
-
- AKSENOV AA, SCHIVO M, BARDAWEEL H, ZRODNIKOV Y, KWAN AM, ZAMURUYEV K, CHEUNG WHK, PEIRANO DJ, DAVIS CE. Volatile Organic Compounds in Human Breath: Biogenic Origin and Point-of-Care Analysis Approaches. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine. 2013:129–154.
-
- ANDERSEN I, JENSEN PL, REED SE, CRAIG JW, PROCTOR DF, ADAMS GK. Induced Rhinovirus Infection under Controlled Exposure to Sulfur-Dioxide. Archives of Environmental Health. 1977;32:120–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
